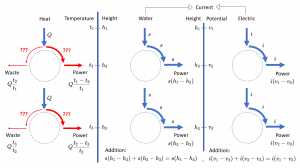
This page is a sub-page of our page on Physics and its Models.
///////
This web page is based on quotes from the book
Physics: The Pioneer Science, Volume I(1): Mechanics, Heat, Sound, by Lloyd W. Taylor
///////
Related KMR-websites:
• …
///////
Other relevant sources of information:
• The Big Misconception About Electricity
• Wavelets: a mathematical microscope
• History of Maxwell’s Equations #1: Gauss’s Law
• Who Invented Wireless: Marconi, Lodge, or Tesla?
• Entropy: Origin of the Second Law of Thermodynamics
• Boltzmann’s Entropy Equation: A History from Clausius to Planck
• What Lies Between a Function and its Derivative? | Fractional Calculus
• What is an Electric Potential?
• The electric and magnetic fields, how electric and magnetic forces arise
• Alternative formulations of Maxwell’s equations
///////
• The Greeks Asked ‘Why’?
• The_Moderns_Asked_’How’?
• The Moderns Exemplify the Scientific Method
///////
/////// Quoting Taylor (Preface page vii)
We physicists do not need to be reminded that we live in what is frequently termed the scientific era. But we are curiously unresponsive to the appeal of general education to show why this is a correct characterization and what its debits and credits are. Unless the sciences live up to this responsibility – and on no other science does it rest as heavily as on physics – society will lose sight of the place of science in the social order.
Let those who smugly maintain that the educational world cannot forget science because society is dependent on science read only a little history to see how easy it is for a section of society unwittingly to cut its own cultural taproot. The American system of specialization in higher education is almost ideally designed to deprive even the educated man of an appreciation of the significance of science in the social order.
There are those who maintain that the interpretation of science is a function of philosophy or of history or of both, rather than of science itself. This has been extensively tried however, and found wanting, partly on account of the lack of an adequate knowledge of science on the part of philosophers and historians. Let those men of science who would delegate this responsibility try to single out from among their non-scientific colleagues those to whom it can be safely entrusted. The result will make it clear that the interpretive responsibility must be discharged by the scientists themselves if it is to have any chance of being done acceptably.
For those who propose a clear opinion that a liberalized course in general physics is preferable to the conventional expository program, the problem of finding class time to introduce the additional subject matter will not be insoluble. Some of the old material will have to be eliminated to make way for it, and each teacher will make his own selections. For the author, no revolutionary changes in class material has been involved. Experience indicates that provision can be made for a measurable acquaintance with the broad bases of scientific doctrine in the very body of scientific instruction itself, indeed rather more effectively so than otherwise. In introducing this element the time involved is not at all proportional to the ground covered, since in the main the process consists of summarizing, from another point of view, material already involved or implied in the traditional course or of merely pointing out implications that would otherwise be missed.
This text may be relied upon to provide most of the guidance which the student will require along such a path, thus leaving the instructor free to conduct his classes in almost the customary manner.
[…]
Part 1: MECHANICS
The further one penetrates into the subject of physics, the more impressed he will be with the central role played by that part of it called Mechanics. Physics is the starting point for all other sciences. It has not only provided the instruments which they use, but has set the very pattern of scientific investigation itself.
In the same sense, mechanics is the starting point of physics. Besides being of importance in its own right, it constitutes the foundation for all the other portions of the subject.
The reputation which physics seems to have acquired, of being a difficult subject, is at least partly due to the necessity of mastering mechanics before proceeding to the other portions which seem more attractive to many. As ordinarily presented, mechanics does present difficulty to the neophyte, primarily because it compels a critical examination and revision of many ideas which the average individual is accustomed to take for granted. If this is considered a disadvantage one should reflect that the degree of such criticism and revision could almost be taken as a measure of the value of any subject in the curriculum.
There is ground for hope, however, that an historical approach to mechanics will enable one to see the subject in terms of the human values out of which it grew and thus enlighten the approach to physics, perhaps thereby making the way less arduous.
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, page 1)
Chapter 1: The Place of Mechanics in the Intellectual Enterprise
Physics as a Curricular Subject (page 1)
[…]
With whatever varying degrees of satisfaction these groups [of students] may pursue their course, there is another reason for the study of physics more valid than any of the foregoing, which under proper guidance can scarcely fail to furnish a contribution worthy of the basic ideal of a liberal education. The greatest significance of the study of physics is that it discloses one of the principal clues to the way men think today. It was in physics that the basic idea – that the physical universe is knowable – had its first development, and it is in physics that that idea is today found in its clearest and most vigorous form.
While the practical aspects of physics are not to be despised, their significance is not so much in the multiplicity of inventions which makes the world so different from what it was a century ago, as in the subtle conception which gave these gadgets birth and which is vastly encouraged by their use – man’s confidence in his intellectual supremacy over nature.
Science as a Unique Discipline (page 2)
When science is viewed in this larger aspect, it is not its effects, profound and far-reaching though they are, that should be the primary interest of the student. It is utterly unique. Literature and the arts have produced principally by special geniuses, and the rate of such production appears neither to grow nor to improve with passing time. Current masterpieces of art and literature are of no greater merit, nor are they being produced any more profusely today in proportion to the population, than the corresponding products of two thousand years ago.
In the sciences, on the other hand, is the first large body of knowledge that is both sequential and cumulative. As a unified army, organized for a sustained assault upon the citadel of human ignorance, there has been nothing to compare with the sciences in the whole recorded development of human thought.
It is possible to question the value of the material which the sciences discover; much of it seems trivial to the lay mind. One may also be fearful of the ultimate effect of scientific philosophy on human welfare; many thoughtful men hold science responsible for some of the major ills of the day. But whether for good or for evil, the fact that science dominates modern thought cannot be disregarded.
Historians have until recently been curiously blind to the scientific idea as a force influencing the trend of world affairs. But there are indications that they are waking from their exclusive preoccupation with imperialistic rivalries and military campaigns to an awareness of what is really the most significant factor in the history of the last three centuries. Thus, Preserved Smith says in his History of Modern Culture (236:1:606):
… whether, as the new salvation or new superstition, science has molded the whole life of the modern world…. All modern production of wealth, all contemporary life, depend on the knowledge of nature acquired by science. But more than that, religion, politics, philosophy, art and literature have capitulated to science, or at least receded before her. There is no department of human activity today untouched with the spirit of experiment and mathematics.
It is of course, possible to live a fairly satisfactory cultural life without having a clear comprehension of the nature and extent of the influence of the scientific idea on human thought. Many people have done so, including, perhaps, most men of science themselves. But since scientific thought has been a dominant influence in our present culture, unfamiliarity with the background and characteristics of scientific thought is sure to constitute a serious blind spot and, for those having the opportunities provided by a liberal arts education, a willful blind spot in one’s world view.
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, page 9)
Chapter 2: The Description of Motion
Physics Began with the Study of Motion (page 9)
Mechanics, in its limited, technical aspect, is concerned with the nature of motions and the conditions of equilibrium. Even in this limited sense, there is not a moment in our lives in which its principles are not being illustrated and utilized in our daily experience. But the science of mechanics has a still broader significance. Its concepts form the logical foundation of all the other sciences. For just this reason, the scientific era had its beginning in studies of motion somewhat over three hundred years ago, and for the same reason, many of the Greek philosophers, two thousand years and more before that, gave a great deal of attention to the nature of motion.
The Greeks Asked “Why”? (page 9)
Though both these groups of intellectual pioneers apparently recognized the basic importance of mechanics, their treatments o the subject were very different. The difference is important because it typifies the distinction between the scientific and the pre-scientific approach to the study of natural phenomena. The Greek philosophers concentrated their attention on the question, Why motion occurs. Aristotle had said that heavy bodies in the vicinity of the earth fell because the earth was their natural place. They possessed the property of gravity which caused them to descend. Light bodies rose because the sky was their natural place. They possessed the property of levity which caused them to rise (17:311a, 311b).
Gravity and levity were thus properties of the bodies themselves, not attributable to anything outside them, such as the earth. For terrestrial bodies, vertical motion up or down was considered “natural,” requiring no force from outside to effect it. For celestial bodies, on the other hand, circular motion was considered “natural.” The sun, moon, and stars described circles because it was their nature to do so. No forces were involved in the production or maintenance of these motions.
Aristotle’s views were revived during the latter part of the Middle Ages and were enshrined in the literature of that time. It is possible that the familiar lines of Robert Seagrave‘s hymn (1742) are attributable to some such source. Certainly they show the impress of a doctrine of “natural places.”
Rivers to the ocean run,
Nor stay in all their course;
Fire, ascending, seeks the sun;
Both speed them to their source.
But the Aristotelian physics took cognizance of other motions than those of rise and fall and of the planets. Besides natural motions there were “violent” motions. These consisted, on the earth, of motions in any other direction than those that had been described as natural, and were characterized by the fact that force was required to effect them, which was not supposed to be true of natural motions. Thus force was required to raise any object naturally carried downward by its gravitation, such as a stone; or to push down any material naturally carried upward by its levitation, such as steam or flame. From the standpoint of later developments, a still more important implication of this doctrine was that force was required to maintain horizontal motion, even in the absence of all friction, since there was no element of naturalness in this type of motion.
The Aristotelian doctrine of natural motions appears rather fanciful to the modern mind. Perhaps it is, but not for the reasons usually invoked. To be sure, it is no longer good physics to postulate the existence of a natural level for every substance; but in other ways, doctrines of “natural states” of one kind or another have a pretty respectable scientific standing. Newton’s first law of motion, for example, implies unequivocally that uniform motion in a straight line is a motion that is natural, in quite the same sense as were the various Aristotelian motions, and that accelerated motion is violent in the sense of requiring a force to maintain acceleration. Isaac Newton, who more than others is responsible for the first formulation of comprehensive laws of motion, did not abandon the idea of naturalness. He merely shifted his point of view somewhat about what was to be considered natural and what was not.
The Moderns asked “How?” (page 10)
But viewed from another angle there is a world of difference between the mechanics of the ancient Greeks and that of the scientific era. The Greeks were obsessed with the question why things moved and concerned themselves not at all with how they moved. It is not so much the errors in their observations of how falling bodies behave, for example, that is impressive, as is the almost complete absence of any observations at all as to how they behave. The scientific attitude, on the other hand, is to concentrate attention exclusively on how things move and to dismiss as a meaningless question any consideration of why they move. This constitutes a radical change in point of view. The scientific question is plainly a much more modest one. Anyone may set up his little instruments and take measurements that will answer, at least in part, the question how an object moves, but to answer the question of why it moves, in the sense that the ancient Greeks and all the later Scholastics asked and attempted to answer it, requires some presumptions about the purposes and the sense of fitness actuating the Almighty Creator which the more humble modern does not feel that he is in a position to make.
Hence, in pursuit of the central quest of mechanics, the first inquiry is how motion may occur. The answer will describe as completely and explicitly as possible the ways in which an object may move from one position to another. Completeness in such an undertaking is, of course, impossible. But to participate in the adventures which explorers of physical science have encountered in studying even the simpler types of motion may give some insight into the nature of the scientific enterprise.
The Moderns Exemplify the Scientific Method (page 11)
As will become evident in a subsequent account of the first scientific study of moving bodies (Chapter 4, pp. 44-45), the distinguishing characteristic of this type of approach to a problem is the analytical or quantitative method: the “method of controlled quantitative investigation” as somewhat cumbersomely phreased by one writer. The idea is important, however phrased, for it distinguishes the scientific from all other types of treatment of a field of knowledge. Its principal aspects are (1) detailed analysis, (2) precise measurement, and (3) mathematical treatment.
Not all subjects of scientific inquiry lend themselves equally to all three of these elements of study, but it is usual to consider that a field of investigation is adaptable to scientific treatment to just the extent that these three techniques are applicable. It is the principal characteristic of physics as a science that , notwithstanding its apparent heterogeneity, almost every phenomenon with which it deals lends itself very readily to analysis, measurement, and mathematical treatment.
A Technical Vocabulary is Necessary (page 11)
Lying at the foundation of all three of these aspects of the scientific method is the necessity for clear concepts and sharp definition of terms. This condition is equally indispensable to analysis of the problem, measurement of the quantities thus analyzed, and mathematical treatment of the data secured. While it is only with the credulous ignorant that impressive words can take the place of sound ideas, it is also true that a carefully constructed vocabulary can contribute greatly to the formation of precise concepts. There is reason to think that for lack of such a vocabulary the birth of physics may have been delayed for at least a century. Some of the fundamental concepts of mechanics are clearly foreshadowed in the writings of Leonardo da Vinci (1452-1519), though their subsequent loss is at least in part attributable to the fact that they were unsupported by an adequate vocabulary. Da Vinci is known principally for his paintings, notably for his Mona Lisa; but the recent discovery of his notebooks, which total some five thousand pages, has made it clear that he anticipated in many respects those who are customarily credited with inaugurating the scientific era. With regard to his handicap of lack of scientific vocabulary, Hart has this to say in his book on The Mechanical Investigations of Leonardo da Vinci (116:76):
“Leonardo suffered badly from the want of a precise and accurate scientific vocabulary…. All of the modern ideas of force, motion, mass, inertia, work, moment, etc., are constantly to be found among the notebooks of our philosopher, but they are clouded by a phraseology which is rarely precise, which is frequently puzzling and which is seldomly rigorous…. The notion of rigor in scientific thought had no place in the fifeteenth century, nor, indeed, in any preceding period.”
[…]
Two Technical Terms: Vector and Scalar (page 18)
The method of polar coordinates is important, not merely because it is useful in specifying positions of points, but because it constitutes an introduction to a concept of still broader utility. There are many entities in physics other than position, the description of which requires the specification of a magnitude and a direction. Thus a motor car is not merely traveling fifty miles an hour; it is also traveling in a certain direction. It thus appears that velocity, like position may be described by a directed line segment. A line of certain length may represent represent the magnitude of the velocity (to which the name speed is often applied). The direction of the line segment then represents that of the velocity. Such a line, limited to the required length, usually possessing a point or “barb” to show which of the two possible interpretations is to be placed on its positive direction, is commonly called a vector, a term that originated by the Irish mathematician W. R. Hamilton in the middle of the nineteenth century. Vectors may obviously be applied to the description of displacements as well as positions and velocities. They may also describe forces, momenta, vibrations, and certain electrical entities. They are thus tools for the treatment of the principal concepts encountered in physics.
In contrast to vector quantities there are scalar quantities (a term also due to Hamilton), those which possess magnitude, but no direction. An example which will come to mind immediately is volume. Such entities require only one number to describe them. Moreover, when two such entities are combined, the result is merely the arithmetical sum of the two. Two weights of four and three pounds respectively add to seven pounds. This is not necessarily true of vectors. In a foregoing illustration, two displacements of four and three miles respectively lodged the traveler at a point, not seven miles from the original point, but five. Similarly a man walking three miles an hour across the width of a ship which is moving forward at four miles an hour would be moving obliquely with reference to the surface of the earth at a rate of five miles an hour. Of course, the resultant, as it is called, of two vectors of magnitude four and three does not necessarily have the magnitude five. It is five only if, as in this case, the two components are at right angles to each other. Its value would be be different from five for any other angle between the two vectors being compounded. For example, if the man walked toward the bow of the ship, the resultant, that is, his actual motion with respect to the surface of the earth, would have the magnitude seven miles per hour, the arithmetical sum of the two components. And if he walked toward the stern, his resultant velocity would have the magnitude of one mile per hour. Methods of combining vectors and of computing their resultants will be illustrated and used in Chapter 6. For the present, a grasp of the two-fold nature of a vector and of the distinction between vectors and scalars will suffice. To bring out these points it has been necessary to digress somewhat from the immediate subject in hand, namely, the general methods of describing position.
Units of length (page 19)
To specify the distance between two positions, some unit of distance must be used. The world’s standard of length, which is also the unit of distance commonly used scientifically, is called the meter. In English units the meter is slightly less than forty inches. It is the distance between two marks on a certain platinum-iridium bar preserved in the International Metric Bureau at Sèvres near Paris. Originally it was intended that this standard of length should be exactly one ten-millionth of the distance between the equator and the pole. But after the meter had been established as the world’s standard of length, more accurate measurements of the size of the earth showed that the length of this standard was not quite the one ten-millionth that it had been supposed. Hence, its original qualification of bearing a simple ratio to the size of the earth had to be abandoned, but the standard remains.
The meter is subdivided decimally and enters decimally into larger units. The most common sub-multiple is the centimeter (one-hundredth of a meter), as the prefix implies, somewhat shorter than a half inch), and the most common multiple is the kilometer (a thousand meters, about six tenth of a mile).
It is unfortunate that the use of the metric system is not yet quite universal, there being two nations, and only two, which have not joined the otherwise complete roster. These are Great Britain and the United States. Everywhere else in the world, including all the nations which are commonly considered “backward,” the metric system has been adopted.
The English and American unit that corresponds to the meter is the yard, being 0.90144 meters. The foot is a third of a yard, and the inch is a twelfth of a foot. The mile is 5280 feet or 1.6094 kilometers.
The Concept of Time (page 20)
In pursuing the course of thought prescribed by mechanics, especially the consideration of motion, a second fundamental concept is involved, that of time. As in the matter of position, it is the measurement of time rather than any speculation as to its nature that is of principal concern. Men of science have been forced to the conclusion that time, like position, is purely relative. The time of an event can be identified only by reference to another event. The terms A.D and B.C. indicate the event to which the so-called Christian nations refer their time. Similarly the year and day, as units of time, originated in the observation of successively repeated events in connection with the sun. There has proved to be sufficient regularity in these events to justify taking them as common units of time.
For long it was felt that the limitation to relative time was a matter of convenience only, and that humanity possessed an intuitive sense of absolute time, or at least of the passage of time, without reference to particular events. Thus Newton expressed this opinion, an analogous one to his opinion of space. He said (190:6):
“Absolute true, mathematical time, of itself and from its nature, without relation to anything external, flows equally; and its other name is duration.”
Somehow Newton failed to see the circularity of this definition. It has become clear since his day that his concept of uniform (“equal”) flow of time was redundant, since it is impossible to give any meaning to such an assertion of uniformity unless the concept of time is already established. Hence Newton’s definition of absolute time was untenable and had to be abandoned. No acceptable substitute has ever been formulated.
The existence of the natural units of time, such as the year and the day, has already been mentioned. Humanity has adjusted its affairs to these units so that they seem natural ones to adopt and use. Unfortunately they are not commensurable and the incommensurability of the artificial unit of the week with both the month and the year adds to the difficulty. Various makeshifts, such as leap year and variable length of the month, have been adopted to obviate the worst conflicts of these units. Since considerations of convenience indicate that one of these units should be considered fundamental, the day has been chosen to perform this rôle. This is the lapse of time involved in successive apparent revolutions of the sun around the earth. But since, by reason of variations of the speed of the earth in its orbit, associated with variations of distance from the sun, successive solar days are not of the same length, the day is taken as the mean or average of these fluctuating periods. The mean solar day is universally subdivided into twenty-four hours, which are further divided into minutes and seconds. The second, the \, \frac {1} {86.400} \, part of a mean solar day, is used by the scientific world as its unit of time.
The Concept of Speed (page 21)
Time is involved in such concepts as speed. A motor car is said to be traveling at the rate of fifty miles per hour. It is thereby implied that if the speed should remain unchanged, the car would in the course of one hour travel a distance of fifty miles, in two hours one hundred miles, and so forth. Such uniformity of speed would be almost impossible for a motor car, though it might be approximated by an airplane. The theoretical possibility of realizing it is, however, one way of giving intelligibility to the concept of instantaneous speed, the logic of which may otherwise appear somewhat troublesome to the beginner, notwithstanding the fact that the mere intuitive concept of instantaneous speed is the common possession of every modern child.
Speed, the rate of traversing distance, may thus be defined as the ration of distance traveled to time elapsed. Not always is the fact that the words “rate” and “ratio” come from the same root so clearly shown. Miles per hour, feet per second, yards per minute: these common expressions, though cumbersome in use, illustrate the basic idea of speed as a ratio of distance to time. Communication would be made easier if names were provided for some of the common units of speed, to replace, for example the awkward expression “miles per hour”; but this has been effected in nautical practice only, in the term knot. The knot, instead of being a unit of distance, the seagoing brother of of the mile, as many landsmen perhaps think, is a unit of speed. It means simply one nautical mile (6080 feet) per hour. For the case of uniform speed (non-uniform speed will be treated in some of the following chapters) a convenient algebraic relation between distance, speed, and time may be deduced from the definition of speed. Since
\, \text {speed} \, = \, \dfrac { \text {distance} } { \text {time} } ,
then
\, \text {distance } \, = \, \text {speed} \times { \text {time} } .
This relation was first formally recorded by the Moslem natural philosophers of the twelfth century (8: 105). Also the obvious remaining permutation of the relation
\, \text {time} \, = \, \dfrac { \text {distance} } { \text {speed} } \,is occationally useful, though less frequently so than the previous two. To express this algebraically, where \, t \, represents time, \, v \, represents speed, and \, s \, represents distance
\, v = \dfrac { s } { t } \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; s = v \, t \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; t = \dfrac { s } { v }. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (1)
In the description of motion, the principal units required are those of length and of time. In addition, the customary methods of identifying position will be needed, as described in this chapter. With the assistance of these concepts, it will be possible to turn to a consideration of the first major undertaking in physics, Galileo’s study of falling bodies.
NOTE: It would seem more natural, perhaps, to use \, s \, for speed and \, d \, for distance. For various reasons, this notation has not commended itself to physicists, and adherence to the accepted notation seems best.
Chapter 4: The Old and the New
“To give us the science of motion, God and Nature have joined hands and created the intellect of Galileo.”
FRA PAULO SARPI, sixteenth century
The Scientific Spirit is Fragile (page 38)
It will be worth while to interrupt the story of the unfolding of the science of mechanics long enough to consider some of the larger aspects of Galileo’s contributions to the growth of knowledge. It is not to his scientific discoveries in other fields that reference is here to be made, though some of them were perhaps of even greater importance. It is rather to the influence that his work had on the intellectual enterprise as a whole, to the way his life modified the prevailing modes of thought, that attention is now to be given.
The period of history termed the scientific era began at about the time of Galileo. This may be and doubtless is in part, a coincidence. Even those who are inclined to give Galileo the maximum degree of credit concede that he lived at an opportune, even a crucial, time. Though to regard his work as a mere symbol of a great change in the prevailing mode of thought certainly understates the case, although even on this basis it would be well worthy of careful examination.
To grasp the real significance of the intellectual revolution (for it was nothing less than that) which had its beginning at about the time of Galileo, one must set it against the background of the era in which it occurred. Such an undertaking, difficult almost to the point of impossibility in a brief account such as this necessarily is, may be rendered somewhat easier by certain analogies from contemporary political developments.
During the first World War and the years immediately following, the fundamental sciences were virtually paralyzed throughout the world. The services of men with scientific and technical training were requisitioned to develop the vast engines of destruction upon the operation of which each of the belligerents considered that its survival depended. During that time the continuance of able-bodied individuals in pursuits such as pure sciences, which were not calculated to give support more or less directly to the military operations of their countries, was regarded with increasing disfavor and by the end of the war had virtually ceased.
The war hysteria of which this was merely a minor example was, of course, even more marked in Europe than in the United States. Post-war economic confusion and distress caused the continuation of this attitude and led to the formation of the various dictatorships or their equivalents, one of the characteristics of which has been not merely the extinction of political liberties, but also the “co-ordination” of all group undertakings, including scientific education and research, in the service of the state. Were it not felt that such activities could be made useful to the state, “co-ordination” would speedily give way to elimination.
A Scientific Götterdämmerung in the Twentieth Century (page 39)
One of the results of this process has been the frontal attack in Germany on the whole idea of disinfected intellectual endeavor and even on the use of reason. At an anniversary of the University of Heidelberg in the summer of 1936, Dr. Bernhard Rust, Nazi Minister of Science and Education, made the following statement of policy:
“The old idea of science based on the sovereign right of abstract intellectual endeavor has gone forever. The new science is quite the opposite of uncontrolled search for truth which has been the ideal heretofore. The true freedom of science is to support the State and share its destiny and to make the search for truth subservient to this aim.”
In 1935, Professor Philipp Lenard, a Nobel prize winner and physicist of high standing in a public address to a gathering met to do him honor stated:
“We must recognize that it is unworthy of a German – and indeed only harmful to him – to be the intellectual follower of a Jew. Natural science properly so-called is of completely Aryan origin and Germans must today also find their own way out into this unknown.”
As in Germany, so in all the other dictator-ridden states, science has become merely a political pawn. Truth is no longer sought in these countries; it has already been determined by Der Führer, Il Duce, or the Central Committee or the Communist Party. By high-pressure propaganda, fragments of truth are stressed and mountains of fiction raised to support these part truths. Nor is the rest of the world entirely free of similar threats. Where the process will end, if indeed it ends at all before it brings another Dark Age, is yet to be seen. Its first fruits are already evident in the European war which began in 1939, prefaced by the “peace” of Munich in 1938.
The Pre-Scientific Intellectual World (page 39)
If such a state of affairs can exist in the twentieth century, with the growth and unanimous acclamation of science as the principal characteristic of the preceding era, it should be no matter for perplexity that in the sixteenth century the scientific ideal met an almost impenetrable wall of inertia and opposition. With nothing in the thousand years preceding to suggest the validity of what we have learned to term the scientific method, the wonder is that it should have gained a foothold at all.
There is however, one great difference between the spirit which oppresses science in the twentieth century and that which tried to bar its way into the intellectual world in the sixteenth. In Galileo’s time the totalitarian policy of discouraging those types of intellectual endeavor not immediately useful to the state had not come into vogue. The value of disinterested reason was not questioned. There were, to be sure, great differences between that time and the present over what was to be considered reasonable. One of those differences will shortly be described.
But reason itself was not only not frowned upon, but was elevated so high that paradoxically it took precedence over observable fact. Instead of being concerned with factual material, securable by experiment, the intellectual endeavors of the sixteenth century were devoted to interpretations of the pronouncements of the Church Fathers and later of the early Greek philosophers, especially Aristotle. Great webs of logic were spun about these writings, by which the authors were made to bear a weight of strained interpretation far beyond anything that they had remotely contemplated. To establish a contention dialectically through this kind of interpretation of the Authorities led the Scholastics, as the intellectuals of that time have now come to be called, into a feeling that no other kind of demonstration possessed any validity. Hence, it came about that experiment, as a means of the acquirement of knowledge, was retarded in coming into its own.
We are so steeped in the prevailing recognition of the validity of experimentation that it is almost impossible to realize that an age of culture and intellectual achievement could have existed without it. Yet such was the case, for the intellectual interest in art, history, and religion which characterized the Italian Renaissance had reached a high stage of development over a course of a full three hundred years before the development of experimental science. Even Scholasticism was an intellectual discipline of the first magnitude, and it may well be that it aided one aspect of the sciences. Indeed it is maintained in some quarters that the forging of the tools of dialectic and precision of expression at the hands of the Scholastics was a necessary prelude to the birth of scientific thought.
“The recognition that physical phenomena tend at least to fall into regular patterns is what makes modern science possible, and this recognition is but an extension of the schoolmen’s contention for realism.”
However this may be, the failure to recognize the possibility of using experimentation to provide substance for forms of logic, otherwise largely lacking in content, deprived the Scholastics of what might otherwise have been the distinction of initiating the scientific era a century or more earlier than it actually came to pass.
No Experiments Allowed (page 41)
The following burlesque, the authorship of which has been attributed to Francis Bacon, is possibly not too unfair a representation of the case of mind of the Scholastics with regard to experimentation.
“In the year of our Lord 1432 there arose a grievous quarrel among the brethren over the number of teeth in the mouth of a horse. For thirteen days the disputation raged without ceasing. All the ancient books and chronicles were fetched out, and wonderful and ponderous erudition such as was never before heard of in this region was made manifest. At the beginning of the fourteenth day a youthful friar of goodly bearing asked his learned superiors for permission to add a word, and straightway, to the wonderment of the disputants, whose deep wisdom he sore vexed, he beseeched them to unbend in a manner coarse and unheard-of and to look in the open mouth of a horse and find answer to their questionings.
At this, their dignity being grievously hurt, they waxed exceeding wroth; and, joining in a mighty uproar, they flew upon him and smote him, hip and thigh, and cast him out forthwith. For, said they, surely Satan hath tempered this bold neophyte to declare unholy and unheard-of ways of finding truth, contrary to all the teachings of the fathers. After many days more of grievous strife, the dove of peace sat on the assembly, and they as one man declaring the problem to be an everlasting mystery because of a grievous dearth of historical and theological evidence thereof, so ordered the same writ down.”
The facts are scarcely less strange than the foregoing fancy. Some years after Galileo studied free fall, he perfected a telescope and with it discovered the moons of Jupiter. Prior to this, great significance had been attributed to the supposed existence of seven planets; namely, the sun, moon, Mercury, Venus, Mars, Jupiter, and Saturn. This was really a type of number mysticism, from which even the greatest scientists of the day were not entirely free. The new satellites, which were regarded as additional planets, destroyed all the supposed significance lent by the magic number seven to the solar system, and aroused the opposition of those whose professional prestige rested in part on the continuance of the old doctrine. Thus Francesco Sizzi, a Florentine astronomer, argued (157:106):
“There are seven windows in the head, two nostrils, two ears, two eyes, and a mouth; so in the heavens there are two favorable stars, two unpropitious, two luminaries and Mercury alone undecided and indifferent. From which and many other similar phenomena of nature such as the seven metals, etc., which it were tedious to enumerate, we gather that the number of planets is necessarily seven…. Besides, the Jews and other ancient nations, as well as modern Europeans, have adopted the division of the week into seven days, and have named them from the seven planets; now if we increase the number of planets, this whole system falls to the ground.”
At about this time, Galileo wrote to Kepler, the famous German astronomer, who was a close friend, the following (157:106):
“Oh my dear Kepler, how I wish that we could have one hearty laugh together! Here at Padua is the principal professor of philosophy, whom I have repeatedly and urgently requested to look at the moon and planets through my glass, which he pertinaciously refuses to do. Why are you not here? What shouts of laughter we should have at this glorious folly! And to hear the professor of philosophy at Pisa laboring before the grand duke, as if with magical incantations to charm the new planets from the sky.”
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, page 323)
Education does not mean teaching people what they do not know. It means teaching them to behave as they do not behave. It is not teaching the youth the shapes of letters and the tricks of numbers, and then leaving them to turn their arithmetic into roguery and their literature into lust.
It means, on the contrary, training them to the perfect exercise and kindly continence of their bodies and souls. It is a painful, continual and difficult work to be done by kindness, by watching, by warning, by precept and by praise, but above all – by example.
JOHN RUSKIN, 1880
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, page 325)
If scientific education today is unsuited for those who are to make science their life work, it is even less suited for those to whom it is merely to be part of a general education. Men of science complain of the lack of a wide appreciation of scientific knowledge; what else can they expect if they offer to the world only the dry bones of knowledge from which the breath has departed?
Nothing could be better adapted than the ordinary school course, with its tedious insistence on bare and uninspiring facts, to kill any rising enthusiasm.
It is most important, certainly, to impress the student with the nature of scientific truth and with the possibility of definite, positive knowledge concerning the material world…. But to insist on the truth of science and to neglect its meaning is to aggravate the evil which we seek to cure….
NORMAN CAMPBELL (Physics, The Elements, Cambridge University Press), 1910
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, page 97)
Pascal’s Principle (page 97)
Another characteristic of fluids, now known as Pascal’s principle, apparently eluded Archimedes though it was implied in his principle. It is commonly stated as follows:
Pressure exerted at any place on a fluid in a closed vessel is transmitted undiminished throughout the fluid and acts at right angles to all surfaces.
Though these are not the words in which the principle was first stated, they constitute a reasonable modern version of the original phraseology. Though the principle bears the name of Blaise Pascal (1623-62) and is stated more clearly in his Traité de Équilibre des Liqueurs than by earlier observers, the first writer to formulate the principle was Giovanni Battista Benedetti in 1585 (32:287-88). It will be recalled that Benedetti was the first to formulate correctly the general concept of the lever arm (page 82). A year later than Benedetti, and probably independently of him, Simon Stevin also formulated Pascal’s principle. Stevin based it, as he did his derivation of the law of the inclined plane (page 65), on the postulate of the impossibility of perpetual motion. This is therefore, another illustration of an idea being “in the air” at a certain stage of scientific development and crystallizing almost simultaneously in the minds of more than one investigator.
Pascal enunciated the principle, in a somewhat verbose but nevertheless clear way, in connection with his description of a device which later became an important industrial tool, the hydraulic press (patented in 1795 by Joseph Bramah). His description and explanation was as follows (165:75 ff.):
If a vessel is full of water, otherwise completely closed, has two openings, one of which is one hundred times as large as the other; by putting in each of these a piston which fits it exactly, a man pushing on the small piston will exert a force equal to that of one hundred men who are pushing on the piston which is one hundred times as large….
It may be added, for greater clearness, that the water is under equal pressure beneath the two pistons; for if one of them has one hundred times more weight than the other, it also touches on hundred times as many parts of the liquid….
The “Hydrostatic Paradox” (page 98)
Pascal’s principle is, as he here hints, merely a corollary of the “continuity and fluidity of the water.” It is, indeed, the principal distinction between fluids and solids, that solids possess elasticity of both size and shape, whereas fluids exhibit elasticity of shape but not of size. Fluids resist any effort to compress them, but not to change merely their shape. From this follows the transmission of pressure in all directions; from this follows also the inability to resist or transmit tangential forces at the boundaries. Consequently fluid pressure must be perpendicular to the surface.
[…]
The Open Manometer (page 99)
The equality of pressures at the bottom of communicating vessels of liquid is sometimes utilized in the determination of the relative densities of two liquids. Suppose, for example, that a glass U-tube (in effect a pair of “Pascal’s vases“) contained water in one sided and mercury in the other. The two liquids would not stand at the same height, but by virtue of the equality of the pressures at the bottom would stand at heights inversely proportional to their densities. Thus a column of water would stand 13.6 times as high as a column of mercury (Fig. 73). In the same way it is possible to compare the densities of any two liquids which do not mix. The method depends on the pressure equilibrium at the bottom between the two columns of liquid in the U-tube or manometer as such a device is termed. The manometer is in fact primarily a pressure-measuring instrument. It is used quite commonly in comparing pressures of two bodies of gas, especially when they are at relatively low pressures, as the name indicates (the first syllable, man-, stems from a Greek root meaning rare or thin).
The Mercurial Barometer (page 100)
Fig. 74 shows how a manometer may be used to measure the pressure exerted by the atmosphere. Mercury is usually used for this purpose. When the air is removed from one side of the manometer by a vacuum pump, the pressure of the atmosphere on the other side forces the mercury up in the evacuated side until it is balanced by the height of this column. Such a balance occurs when the difference of the heights of the mercury on the two sides is between 72 and 76 cms. under conditions usually obtaining (weather and altitude). When used in this way, the manometer is termed a barometer, meaning pressure-gauge.
The first barometer on record was made in 1644 by Evangelista Torricelli, who had been a pupil of Galileo: Its importance lies not so much in the device itself as in the fact that it was an outgrowth of the first correct view of the nature of atmospheric pressure and of a vacuum. Torricelli’s account, a portion of which is reproduced here, appears in his Collected Works (165:71-72):
“We live immersed at the bottom of a sea of elemental air, which by experiment undoubtedly has weight, and so much weight that the densest air in the neighborhood of the surface of the earth weighs about one four-hundredth part of the weight of water. Certain authors have observed after twilight that the vaporous and visible air rises above us to a height of fifty or fifty-four miles, bit I do not think it is so much, because I can show that the vacuum ought to offer a much greater resistance than it does, unless we use the argument that the weight which Galileo assigned applies to the lowest atmosphere, where men and animals live, but that on the peaks of high mountains the air begins to be more pure (rare) and to weigh much less than the four-hundredth part of the weight of water. [Pascal made a brilliant verification of this speculation of Torricelli four years later.]
We have made many vessels of glass like those shown in A and B [Fig. 76] and with tubes two cubits long. [One cubit was about 58 cms.] These were filled with quicksilver, the open end was closed with the finger, and they were then inverted in a vessel where there was quicksilver C; then we saw that an empty space was formed and that nothing happened in this vessel where this space was formed; the tube between A and D remained always full to the height of a cubit and a quarter and an inch over (75 cms.)…. I assert that (the force holding up the quicksilver) is external and comes from without. On the surface of the liquid which is within the bowl there rests the weight of a height of fifty miles of air; then what wonder is it if into int the vessel CE, in which the quicksilver has no inclination and no repugnance, not even the slightest, to being there, it should enter and rise in a column high enough to make equilibrium with the weight of the external air which forces it up? Water also in a similar tube, though a much longer one, will rise to about 18 cubits, that is, as much more than quicksilver does as quicksilver is heavier than water, so as to be in equilibrium with the same cause which acts on the one and on the other.”
Pascal’s Study of the Vacuum (page 101)
In 1647 Pascal took up the investigation [of the vacuum] where Torricelli had left it. It had, in the meantime, become a highly controversial subject. The controversy originated in the insistence of the Scholastics on the Aristotelian doctrine of the practical and logical impossibility of a vacuum. The rallying cry of this school of thought was “Natura abhorret vacuum.” As a biographer of Pascal remarks (36:49):
“The error was reinforced by the verbalism that cursed scholastic argument. Vacuum, void, emptiness, was identified with Nothing, nihil. Descartes said that if everything should be removed from a vessel, the sides must immediately touch for a vessel cannot be filled with Nothing; that is a logical impossibility, ergo false. The universe, said the thinkers, would sooner fall to pieces than permit an abhorred Nothing in its midst.”
Even Galieo had been only mildly ironical about this Aristotelian doctrine. Having observed, prior to the Torricellian experiment, that (101:16)
“it was not possible, either by a pump or by any other machine working on the principle of attraction to lift water a hair’s breadth above eighteen cubits,”
he is said to have remarked that “Nature’s abhorrence of a vacuum seemed to be limited to eighteen cubits of water,” but to have gone little further with the problem.
Having become convinced of the correctness of Torricelli’s conclusions Pascal leaped into the controversy, invoking a series of striking experimental demonstrations to support his point of view. He was fortunate in living in Rouen, the site of one of the best glassworks in Europe, because he was thus enabled to conduct experiments which would otherwise have been impossible. His biographer, Bishop, describes his demonstrations as follows (36:53):
“In January and February of 1647 he gave some public demonstrations in Rouen before an admiring audience of five hundred of the city’s Eminences. The “plenists” alleged that the apparent void in the Torricellian tubes was in fact filled with rarefied air, which expanded to fill the vacant space. To test this theory, Pascal performed experiments with tubes of varying diameters, in which the space left for the void was now bulging, now narrow. He inclined his tubes until the empty space disappeared; he raised them again to the perpendicular recreating the emptiness. He put a bladder inside the top of his tube; the bladder strained to fill the empty space. In every case the height of the mercury remained constant, while the empty space varied prodigiously. How could the plenists explain the variation in the rarefaction of air?
Did some say that the space was filled with the vapors of mercury? Pascal, with a fine sense of the dramatic, performed a striking experiment. He displayed, in the glassworks yard, two tubes, 46 feet long, bound to ships masts, which were mounted on pivots at their mid points. He filled one tube with water, reversed it so that the open end sat in a tube of water, and removed the stopper.. The water fell to a height of 34 feet above the water level in the tub. He asked his plenist friends what would happen if wine should be used in the test. They replied that wine, being more volatile than water, would set free more vapors, and would descend farther in the tube. Pascal filled one of his tubes with red wine and the other with water; the wine stood higher in the tube than did the water.
He exhibited a siphon, with one arm 50 feet high, the other 45 feet. The apparatus stood as high as a fifth-story window. He proved that the siphoning of the water did not take place when the connecting arm stood more than 34 feet above the water. When the apparatus was slowly tipped sideways, the siphoning could begin again when the connecting arm came to 34 feet above the water.”
The Effect of Altitude on Barometric Pressure (page 103)
Pascal supplemented the foregoing with a number of other equally ingenious and sensational demonstrations, including the use of a plunger-type syringe as a vacuum pump. This was the first time a mechanical pump had ever been used to produce a vacuum. It furnished Otto von Guericke with a tool which he used most effectively a quarter of a century later.
But the horror vacui died hard. Pascal capped his contributions to the controversy by his famous experiment on an adjacent mountain, Puy-de-Dôme, an experiment which had been dimly foreseen by Torricelli. Being a semi-invalid, he was not able to make the ascent himself, but enlisted his brother-in-law, Florin Perier in a letter of November 1647 (36:64):
“If it happens that the mercury is less at the top than at the bottom of the mountain (as I have many reasons to believe, although all those who have meditated on this matter are contrary to this belief), it will necessarily follow that the weight and pressure of the air is the only cause of this suspension of the mercury, and not the horror of the void, since it is very certain that there is much more air to weigh at the foot of the mountain than at its summit; while one cannot say that Nature abhorrs the void more at the foot of the mountain than at its top.”
The proposed experiment was carried out in September 1648. In a letter to Pascal, Perier reported that at the top of the mountain (165:74)
“it was found that there remained in the tube no more than twenty-three inches two lines (a line being a twelfth of an inch) of quicksilver, whereas at the Minimes there was found in the same tube a height of twenty-six inches three lines and a half.”
The observations were repeated at several stages on the party’s return. The mercury rose in proportion to the loss of altitude. And when they reached the point of departure they learned that a second barometer which they had left in the care of an observer as a control, had not varied at all during their absence.
Pascal was greatly delighted with the results of the experiment. After checking them by similar tests at the old tower of Saint-Jacques-de-la-Boucherie, where a statue of Pascal was later erected to commemorate the event, as well as at other accessible high buildings, he published a record of the exploit under the title An Account of the Great Experiment of the Equilibrium of Liquids. In conclusion he says, in part (36:66)
“Nature has no repugnance for the Void; she makes no effort to avoid it. All the effects that have been attributed to this horror proceed from the weight and pressure of the air, and that is the sole and veritable cause; it is from ignorance of it that that imaginary horror of the void was invented, to make an explanation. This is not the only circumstance wherein, mans weakness having failed to find the true causes, he has explained (the causes) by specious names which fill the ears and not the mind.”
One could wish that the practice which Pascal here castigates had gone out of vogue along with the doctrine of horror vacui.
Among Pascal’s comments on the possible utility of the barometer are references to the two principal uses which are made of it today. In the foregoing report he mentions that here is now an accurate and convenient means of measuring altitude. In some papers found after his death fourteen years later is a set of observations on the variations of the height of the Torricellian tube according to the weather, the first account of this modern use of the barometer. He says (36:671):
“This knowledge can be very useful to farmers, travellers, etc., to learn the present state of the weather, and that which is to follow immediately.”
The Genesis of Boyle’s Law (page 105)
Among other contributions, Pascal made what was then the novel observation that air was compressible. He stated that he had observed this by noting the gradual distension of a balloon which, originally only partially inflated, was then carried to the top of Puy-de-Dôme. It is almost inconceivable that, with all his experiments with manometers, he should not have encountered the compressibility of air in the same manner that Robert Boyle did in some experiments performed only a few years after the climax of Pascal’s work in this field. But if he did, he left no record of it. That distinction goes to Robert Boyle (1627-91).
In a book published in 1660, which labored under the cumbersome title, A Defense of the Doctrine Touching the Spring and Weight of the Air, Boyle set forth the relation the relation between the pressure and volume of air, the substance of what is now known as Boyle’s law. Everyone who has had experience pumping u a tire is well aware that the application of pressure reduces the volume of air. Boyle’s law states the relation between pressure and volume of a given mass of air as that of inverse proportionality, that is,
\, p \, \propto \, \dfrac {1} {v} .
Introducing a constant of proportionality, \, c , to substitute an equality for the proportionality
\, p = \dfrac {c} {v} , whence \, p v = c. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (6)
To verify this hypothesis “that supposes the pressures and expansions to be in reciprocal proportion,” Boyle made use of a manometer, one end of which was closed, mercury being poured into the open end. The air entrapped in the closed side was compressed by the weight of mercury. Boyle (165:85)
“continued this pouring in of quicksilver till the air in the shorter leg was by condensation reduced to take up half the space it possessed (I say possessed, not filled) before; we cast our eyes upon the longer leg of the glass on which was likewise pasted a list of paper, carefully divided into inches and parts, and we observed, not without delight and satisfaction that the quicksilver in that longer part of the tube was 29 inches higher than the other. For … the air was (previously) able to counterbalance and resist the pressure of a mercurial cylinder of about 29 inches, as we are taught by Torricellian experiment; so here the same air being brought to a degree of density about twice as great as it had before, obtains a spring (of 29 additional inches) twice as strong as formerly.”
A Stricture on Boyle’s Law (page 106)
Thus Robert Boyle verified his hypothesis that “the pressure and expansion are in reciprocal proportions.” His work apparently did not receive the attention that it merited, for a quarter of a century later Edme Mariotte (1620-84), not knowing of Boyle’s work, rediscovered the same law, which now goes by his name nearly as commonly as it does by Boyle’s. Mariotte made one qualification which is important, and which Boyle, though he must have known and allowed for it in his experimentation, does not explicitly mention. Mariotte remarks, rather incidentally, that
“The air also dilates very easily by heat and condenses by cold, as we can notice any day by experiment.”
Thus the pressure of an enclosed gas will fluctuate with temperature, besides the changes in pressure that may attend any changes of volume that are brought about. Unless changes of temperature are guarded against, the inverse proportionality between pressure and volume in a gas discovered by Boyle and Mariotte will not be accurately registered. In other words, Boyle’s law obtains only for constant temperatures. The actual study of the effect of changing temperature on gas was, rather surprisingly, not made for more than a century.
It is common to represent Boyle’s law graphically. If values of \, p \, in equation (6) be represented as ordinates with \, v \, as abscissas for a given value of \, c , a curve similar to Fig. 81 results. This curve has the form of a hyperbola. Boyle’s law was first represented in 1686 in this way by Edmond Halley, the friend who published Newton’s Principia at his own expense.
Other Limitations on Boyle’s Law (page 108)
It is appropriate to inquire into the limits of the validity of Boyle’s law. Is pressure inversely proportional to volume for all gases, through all ranges of pressure and temperature? A part of the answer is to be found in data which Boyle himself took. After his first manometer had been broken by accident, he made another, the open side of which was more than eight feet in length. The pressures in this tube were found to mount more rapidly than the inverse proportion allowed for. Subsequent observers have verified this fact. The discrepancy vindicates Boyle’s law, however, rather than invalidating it; this for two reasons.
First, the effective, unoccupied volume of a container is not its entire interior, but is less than the entire interior by the actual volume occupied by the molecules of the gas. The free space which the molecules have for their motion is thus smaller than the actual volume of the container.
Second, the external pressure is not the only agency holding the gas molecules together. There is a certain gravitational attraction between the molecules themselves which aids the external pressure in this respect.
When allowance is made for these two effects, as is done when the so-called Van der Waals’ equation is substituted for Boyle’s law, the discrepancies shown by the data of Boyle and his successors disappear. Boyle’s law is sometimes said to apply rigorously only to “perfect” gases; that is, gases whose molecules have no volume and which do not exert any forces on each other except during the instants of actual impact.
These two disturbing factors become prominent in a gas as the temperature approaches the point of liquefaction. In fact the liquid state obtains when the forces of attraction between molecules are great enough so that no external pressure is required to prevent expansion. Hence, Boyle’s law applies most closely to gases whose “boiling points,” that is, temperatures of transition from the liquid to the gaseous state, are very low, such as hydrogen, nitrogen, oxygen, and the so-called noble gases, helium, argon, etc., and applies much less closely to steam, to the gases used in mechanical refrigerators, or to any gas not at a temperature far above the point of liquefaction.
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, p. 217)
The Conservation of Mechanical Energy
A Famous Controversy (page 217)
Late in the seventeenth century a curious dispute arose in scientific circles. Newton had taken “quantity of motion” to be measured by the product of mass by velocity. Descartes had still earlier adopted the idea and written extensively in that vain, though his writings were somewhat confused and lacked the precision of Newton’s treatment of mechanics.
Leibniz, who has already been introduced as having been the first to formulate the concept which ultimately developed into that of kinetic energy, took issue with Descartes. He insisted that a more appropriate measure of the quantity of motion would be the product of the mass by the square of the velocity.
It is important to envisage exactly the point at issue in this famous controversy. In modern terminology Descartes’ argument was as follows (67:135): If you wish to compare two forces \, f \, and \, F \, , allow them to act for any given time \, t \, , upon two masses \, m \, and \, M \, respectively; then the ratio of these forces will be
\, \dfrac {f}{F} = \dfrac {ma} {MA} = \dfrac {mat} {MAt} = \dfrac {mv} {MV}, \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; \, (1)
that is, as the ratio of the resulting momenta.
Leibniz, on the other hand, based his opinion upon the common experience that it requires the same “force” (as he called it) to raise a body weighing \, m \, pounds through a height of \, 4h \, feet as it does to raise a body of \, 4m \, pounds through a height of \, h \, feet. Now it is well known, argued Leibniz, that a body in falling through \, 4h \, feet acquires a velocity just twice as great as when it falls through \, h \, feet. Hence, if the “force” required is the same in each of the two cases just mentioned, this “force” must be measured by the product of the “body” (mass) by the square of the speed. In symbols, letting \, s \, be the given distance through which the body is moved,
\, \dfrac {f}{F} = \dfrac {ma} {MA} = \dfrac {mas} {MAs}. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; \, (2)
But for uniform acceleration
\, v = \sqrt {2as} \, (see page 29).
Hence, the ratio of the forces becomes
\, \dfrac {f}{F} = \dfrac { \frac {1}{2} m v^2 } { \frac {1}{2} M V^2 } = \dfrac {m v^2} {M V^2}. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\, (3)
Thus the issue was squarely joined between the two men. Leibniz fired the opening broadside in 1686 by the publication of a two-page treatise bearing the long title (152:3:180):
A short Demonstration of a Remarkable Error of Descartes & Others, Concerning the Natural Law by which they think the Creator always preserves the same Quantity of Motion; by which, however, the Science of Mechanics is totally perverted.
The subsequent dispute raged between the men and their participants for more than half a century. It was finally settled by d’Alembert in 1743 (6:xvii) when he showed that the contest had been a mere battle of words. Thinking that they were talking about the same thing, the disputants had actually been thinking about different things. Both were right, each in a separate field. Descartes’ adoption of momentum as the measure of quantity of motion was correct for a force acting for a certain time. Leibniz’s adoption of vis viva (as he called \, m v^2 ) as the measure of quantity of motion was correct for a force acting through a given distance. Each had its use, and once it was clear that momentum and vis viva were two entirely separate entities, no further occasion for disagreement existed.
The fact is – though nobody up to the time of d’Alembert had realized it – that mechanics had been from the beginning confronted with the necessity, not of choosing between these alternative points of view, but of incorporating both of them into its structure. Until it did so, thoroughly and completely, it was but half a science, limping along on one leg while its normal logical progress required two.
The Gradual Emergence of the Energy Principle (page 218)
Unknown, even to the pioneers of science themselves, the energy principle had been lurking in the hiding-places of mechanics for a long time. It had been employed by Huygens in his Horologium Oscillatorium, though with what degree of appreciation of its generality is hard to say. It had also been used unconsciously by Galileo, and it was even implied in the work of Archimedes on the balance. Great as was the work of these men, we now realize that they were in large measure blind to what is possibly the most fundamental single principle in mechanics.
That a pendulum, disregarding the small effects of friction and air resistance, swings as far beyond the vertical on one side as it starts from the vertical on the other side is a fact of common observation. Galileo pointed out (101:170) that the equality of the distances on the two sides of the vertical was not the essential point, but rather the equality of the heights before the swing and at its completion. He illustrated his point by interrupting the swing of a pendulum started at C (Fig. 123), by allowing the string to strike a small nail at E. From this point on, the pendulum acted as if its length were EB, and hence swung through the arc BG. The point G reached by the pendulum Galileo observed to be on the same level as C, from which it started, and as D, which it would have reached in a normal swing. This was also true if the nail were placed still lower, as at F. Thus in all cases the pendulum bob rose to its initial height. The experiment failed only
“when the nail is placed so low that the remainder of the thread below it will not reach to the height CD;… then the thread leaps over the nail and twists itself about it.”
This equality of initial and final heights was true not merely of pendulums, but of all bodies subject to gravity and moving under constraints which cased them to fall and rise. It was true, for example, for balls that rolled down one incline and up another, except for the disturbance introduced by the discontinuous junction of the two inclines. In fact, Galileo made his original application of the idea of equality of heights to the case of inclined planes and he resorted to the pendulum principally to escape this disturbance.
Now, Galileo had already observed that velocities along inclines depended solely on the vertical height through which the descending bodies had passed (see page 29 ff.). He did not, however, connect these heights with the squares of the velocities associated with them, nor, as has already been observed, had he formed any concept of mass. Thus he was far from being in a position to recognize, or even remotely conceive, the principle of energy applied in his pendulum and inclined plane experiments.
This omission Leibniz supplied, though in a somewhat preliminary and restricted form. The value of his contribution did not lie so much in his recognizing the significance of the square of the velocity for bodies moving under gravity – Huygens had already done that (see page 29) – nor even his discovering that the product of mass by square of velocity was one measure of quantity of motion. It lay rather in associating this product with the “force” as he called it, required to raise a body to a level such that it could develop this vis viva in falling. That is, he found that the possession of a “force” in the form of vis viva involved the prior exertion of an equal “force” sufficient to impart it. Whether this were exerted directly on the body, thus setting it in motion, or were expended in raising the body, after which it required its velocity by falling, was of no significance. To adopt the latter alternative, however, may help in correlating Leibniz’ concept with Galileo’s experiment.
Work as a Concept of Physics (page 220)
Leibniz had commented on the equivalence between raising a mass \, m \, through a height of \, 4h \, and raising a mass of \, 4m \, through a height of \, h . The significant point in his mind was thus the product of the force exerted and the distance traversed in the direction that the force acted. This product of force by distance is now known as work, in the technical or scientific sense; Leibniz called it “force.”
Leibnitz’s selection of this term was most unfortunate. The product of force by distance, whatever it may be, cannot properly receive the same name as one of its factors.
The word “force” has always been a much overworked term. This is true even today, as reference to any dictionary will show. But today it is usually possible to tell from the context whether the word is being used in the technical, Newtonian sense, or in one of its more loosely specified connotations. In the time of Leibniz and Newton this was not true. The technical concept of force as we know it now was struggling for recognition. The very derivation of the word force (from a Greek root meaning muscle or tendon) fitted it excellently for use in connection with this concept. Newton’s use of the word in this natural sense in the Principia went far toward the clarification of the technical vocabulary of mechanics.
Leibniz’s contemporary introduction of the same word in an etymologically illegitimate sense seriously muddied the waters of scientific thought. It took German science two hundred years to clear up the confusion resulting from this use of one word for two different fundamental scientific concepts, and the handicap to physical science and philosophy in other countries was almost as serious. This confusion is the more regrettable in that force and work had been clearly distinguished two hundred years before Leibniz by Leonardo da Vinci, who had remarked that work implied a distance moved in the direction of the force (153:50).
Fortunately that time is past. For Leibnizian “force” the term work has now been substituted, as defined above. Perhaps it is unnecessary to give further emphasis to the statement that it is a technical term and as such must be sharply differentiated from the same word used non-technically. In the literature of physics, work means just one thing, force multiplied by the distance traveled in the direction of the force. Algebraically,
\, W = f s. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (4)
A man who stands for half an hour holding a fifty-pound weight may feel that he has done some hard work. Judged by fatigue he has. But fatigue is not an element in the physics definition of work. Of the two elements, force and distance-in-the-direction-of-the-force, the second has the value of zero for this case; hence, the product is zero, and, hence, from the standpoint of physics the man has done no work. A certain additional element of plausibility may be attached to this illustration by observing that the man might have placed his fifty-pound load on a box and gone his way. No one would then have been tempted to assert that the box was doing any work, and so it may seem more reasonable to assert that neither did the man. The argument, however, is somewhat specious, and one is on firmer ground to revert always to the fundamental definition rather than to resort to such plausible parallels.
Just as the physiological measure of work (fatigue) is inadmissible in physics, so is also the industrial criterion. However useful industrially a man’s labor in factory, store, or office may be, from the point of view of physics the work which he performs is measured only by the product of the forces he exerts and the distances which he simultaneously travels in the direction of the forces.
It is sometimes said that the advantage of the physics definition of work is that it involves measurable terms, force and distance, whereas other criteria, such as the physiological and the industrial, are not capable of measurement. Whatever validity this argument may have had in the past, it is largely irrelevant at present. Physiological states are capable of accurate instrumental identification now by such means as oxygen consumption and hydrogen-ion concentration of the blood, and efficiency experts have devised dishearteningly accurate ways of measuring the effectiveness of labor.
It is not its measurability that determines the scientific validity of any entity, though measurability is a useful technique. But besides this, concepts must be logically necessary in the scientific scheme before they may be counted among the foundation stones of the pure sciences. Neither fatigue nor industrial value are necessary in this way; hence, the fact that they may have been rendered measurable with the aid of scientific instruments does not entitle them to a place among scientific concepts. But of this more later.
Work, being defined as the product of force by distance, is expressible in corresponding units. A common English unit of work is the foot-pound, lifting one pound through a distance of one foot against gravity, or one half-pound through two feet, or any similar product of distance in feet by force in pounds which gives the value unity. This is, however, a somewhat unsatisfactory unit, since the gravitational attraction upon a given mass is measurably different for different localities.
The difficulty is avoided by taking advantage of the so-called absolute system of units in common scientific use – involving the metric system, with the meter as the unit of length and the newton as the unit of force (see page 128). This metric unit of work, which would otherwise have to go by the awkward name of meter-newton, has been given the name joule, in honor of James Prescott Joule (1818-69) of Manchester, England, whose contributions will be encountered in the study of heat.
It is now possible to combine Leibniz’s idea about vis viva with Galileo’s association between height and velocity. The combination results in the concept, not fully realized until modern times, that when a body is raised to a certain height and then allowed to fall back to the original level, the work done upon it against gravity appears in the form of kinetic energy. This is true whether the descent is vertical, along the arc of a circle, along a straight incline, or in any other path as long as the same height is involved in every case and frictional losses are negligible. Moreover, it applies equally to the case, illustrated by accelerating a bicycle or an automobile, of applying a force through a certain distance and thereby producing velocity directly.
So the idea of conservation of energy, formulated by Huygens for elastic impact and in this instance applied only to kinetic energy, applies in a somewhat modified form to any case involving the conversion of work into kinetic energy. This is a considerable extension of the conservation principle, though, as will be seen later, it is only a preliminary step in the long course that the principle of conservation of energy is ultimately to travel. It is momentous, however, as first steps always are, and merits a close examination.
The Idea of Potential Energy (page 222)
If we state that a falling body is converting into kinetic energy the work which was previously done upon it in the process of raising it, we imply that the body thus raised must somehow possess, before it starts to fall, the energy which is later to appear in kinetic form. Once the concept of energy is formed, the possession of energy by a moving body is pretty obvious. Kinetic energy under the name of vis viva thus came early into the scientific scheme. But the possession of additional energy by an object which has been raised from a lower to a higher level is considerably less obvious. It is, in fact, a rather subtle concept. It was a concept which eluded both Leibniz and Descartes. They thus had at least this much in common – notwithstanding their pronounced differences of opinion on energy of motion.
The first time that the idea appeared that a stationary object could possess energy by reason of its position was in 1803 in a book on mechanics by L. N. M. Carnot (1754-1803) (59:37). In that book he says:
“Vis viva can figure either as the product of a moving power and a length or a height. In the first case it is a vis viva properly called; in the second it is a latent vis viva.”
During the next fifty years a whole series of names came into use for this entity which Carnot had termed latent vis viva, and one by one they dropped out of use (122:13). Finally, in 1853, the name potential energy, first used by W. J. M. Rankine (1820-72) (219:203), found general favor and has been used ever since.
Hence, from the welter of scientific confusion about the nature of energy two fundamental concepts emerge: kinetic energy and potential energy. The former is energy possessed by reason of motion; the patter is energy possessed by reason of position or condition, as in a raised weight or a stretched spring. It will be clear that either kind may come into being in consequence of the performance of work; in the first case, work devoted to producing speed; in the second case, work involving some reversible process which subsequently can be made to yield up the energy thus stored.
[…]
Newton and the Energy Principle (page 228)
Newton apparently had a glimmering of a certain restricted portion of the energy principle, though, he never made much use of it. At the close of a long scholium on his third law of motion he says (190:28):
“For if we estimate the action of the agent from the product of its force and velocity and likewise the action of the impediment from the product of the velocity of its several parts and the forces of resistance arising from the friction, cohesion, weight and acceleration of these parts, the action and reaction in the use of all sorts of machines will be found always equal to one another.”
Some writers (for instance, 250:33-38) have seen in this a complete prevision of the energy principle, but such an attitude reads more into Newton’s statement than is in any way justified by the text of it. The concept of kinetic energy is entirely absent, and even the concept of work, which is its central point, is only in part suggested, since he talks about product of force and velocity, instead of product of force and distance. It also, like the concepts of Huygens, Leibniz, and Descartes, lacks another element of generality, an explanation of which would necessitate some of the concepts involved in the study of heat. In fact, Newton seems to have had no deeper insight on this point than may be attributed to Galileo a generation before him.
[…]
Power (Page 231)
There is a final application of the concept of work to be made before the next subject is taken up. While the same amount of work would be required, say, to ascend a light of stairs in five minutes and in five seconds, the rate of doing work would be quite different in the two cases. The rate of doing work is of sufficient importance to have received a name and a unit.
Rate of doing work is termed power. In this case as in the case of other technical terms careful attention must be paid to the exactness of this definition, and how it contrasts with the wide range of vague meanings attached to it in non-technical use. Power is work done per unit time. In the English system the foot-pound has been mentioned (page 222) as a unit of work.
Thomas Savery, whose pumping engine, patented in 1698, was the first device actually to use steam power in industry, suggested as a standard of power the rate at which a horse could do work. Careful measurements of this rate were made by James Watt (1736-1819), who concluded that an average draught horse would exert steadily a 150-pound force while walking at the rate of 2 1/2 miles per hour and, hence that a horse could perform work at the rate of 33,000 foot-pounds per minute, or 550 foot-pounds per second. This rate was then defined as the horse-power. This is an awkward unit in more ways than one, but it was a natural one to introduce at a time when every prospective customer of the engine-builder was asking the question, “If I buy one of your engines, how many horses will it replace?
In the metric system the unit of power is, naturally, one joule per second, and it is named the watt, in honor of James Watt, whose inventions improved the steam engine nearly to the plane of its present performance. The watt, as a unit of power, is used so commonly in connection with electrical devices that the association produces the impression that it is an electrical unit. Such is, however, not the case. It is fundamentally a unit of mechanical power, similar to the horse-power, though much smaller. It requires 736 watts to be the equivalent of one horse-power. The metric unit of power which is analogous to the horse-power is the kilowatt (1000 watts). The horse-power is thus 0.746 of a kilowatt.
//// FORTSÄTT HÄR
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, p. 249)
Early Speculation on Heat (page 249)
The question “What is heat?” must have been proposed and pondered over by many inquiring minds from the earliest times. Man could not go on forever using fire to cook his food and warm his body without seeking to know something of the source and nature of this agent. The inquiring mind cannot rest satisfied with the mere observation of the facts of nature, but is irresistibly led to investigate their origin and cause. The fact of highest interest and importance is that the sun warms and illuminates the earth, and the questions which must have presented themselves earliest to the attention of philosophers were, “What is heat?” and “What is light?”
The parallel question, “What is sound?” is of a much simpler order, and that any satisfactory answer has been obtained to the two former is probably owing to the proposition and solution of the latter. Amid the phenomena of sound we deal with a medium which we can subject to experiment, and whose properties we can thoroughly examine; but in the phenomena of heat and light we step at once into the sanctuary of the unknown. From the domain of the visible and tangible we pass into that of the invisible and intangible.
The known process, however, gives direction to the line of thought, and, reasoning by analogy, the imagination expands from the domain of the senses and embraces in thought the regions which lie beyond it. By observation and experiment, the human mind becomes acquainted with a knowledge of the properties and relations of things, and, reasoning upon the information thus supplied, we rise to the explanation of the unknown and intangible by means of the ideas which we have gained from what is known and tangible (212:4).
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, p. 250)
The First Temperature-Indicating Instrument (page 250)
Galileo’s thermoscope was the first of the modern developments in the science of heat. Curiously enough there is no statement in his own writings that he ever made it, but one of his students, in a letter written in 1638, explicitly states that Galileo made such an instrument and also makes a sketch of it. His description of it is as follows (277:83):
Galileo took a glass vessel about the size of a hen’s egg, fitted to a tube the width of a straw and about two spans long; he heated the glass bulb in his hands and turned the glass upside down so that the tube dipped in water contained in another vessel. As soon as the ball cooled down the water rose in the tube to the height of a span above the level in the vessel. This instrument he used to investigate the degrees of heat and cold.
Such a thermometer, easily made, will be found surprisingly sensitive. Galileo apparently used it merely to indicate whether temperatures were rising or falling. The scale which he presumably attached to it could scarcely have served any other purpose (in his Dialogues he speaks of 6, 9, and 10 degrees of heat). A scale in the modern sense would have been of little use, since the reading of the instrument depended upon atmospheric pressure as well as upon temperature.
But neither the existence of this pressure nor its variations were recognized at the time. These were the discoveries of Torricelli and Pascal (pages 100, 105). Galileo must have wondered why his thermoscope behaved so erratically. It was, indeed, fundamentally a barometer which was also sensitive to changes of temperature.
Thermometers (page 251)
There is some significance to the fact that scientific comprehension of heat phenomena began when the first instrument of measuring temperature was devised. It illustrates a point which was emphasized early in our study of mechanics (page 45), where Kelvin was quoted as saying,
when you can measure what you are speaking about and express it in numbers, you know something about it….
Galileo’s crude thermoscope was not itself capable of translating temperatures into numbers, to be sure. But it was the parent of instruments that did. Prior to that the only method of judging temperature was by sensation. If anyone needs an illustration of the unreliability of this method, he can perform a very old and very simple experiment first suggested by John Locke in 1690 (155:1:177). Let two hands be immersed, one in hot water, the other in cold. Then let both hands be placed in water of intermediate temperature. This will seem cool to the first hand and warm to the second. Without a more objective (and preferably numerical) measure, judgements of temperature might be quite misleading.
It is the principal function of scientific instruments to provide such objective measures. Without them there could be little agreement on orders of magnitude, and there could be no precise measurement, which is simply another way of saying that there could be no exact science.
It is characteristic of scientific instruments that the thermometer gives indirect information. It utilizes one of the incidental accompaniments of rising temperature, namely, expansion of the materials which participate in the rise of temperature, to give more reliable information than direct sensation would do. Though there are now in use several other methods of measuring temperature, the phenomenon of thermal expansion and contraction constituted the working principle of all the early thermometers and is that of most of those in common use today.
Between 1592 and 1742, just a century and a half, the thermometer developed into its present form. The second stage of its development, in 1632, consisted of inverting the instrument and filling it with water, which thus became the new thermometric substance. Though far less sensitive than Galileo’s form, it no longer responded to fluctuations in barometric pressure, even though the tube was open at the top. Twenty-five years later, the tubes were commonly sealed at the top and alcohol was substituted for water. The first use of mercury was in 1659 (58:100). It had the advantage of being opaque, of not wetting the tube, of conducting heat readily, of having a low freezing point and a high boiling point, and, as was discovered later, of changing its volume more nearly in proportion to changes of temperature than other liquids in common use. It is little wonder that one physicist enthusiastically exclaimed, “Surely nature has given us this mineral for the making of thermometers” (58:117).
Thermometric Scales (page 252)
The growth of thermometric scales, the final stage in the development of the thermometer, was a chaotic process. A commentator in 1779 enumerated nineteen different scales which were in current use. Three have survived: the fahrenheit, devised by a German of that name about 1724 and used mainly in English-speaking countries; the réaumur, devised by a Frenchman so named in 1730 and used mainly in Germany; and the centigrade, devised by a Swede named Celsius in 1742 and used popularly in France. The latter is the scale that is used the world over for scientific work.
The three scales are represented in Fig. 137. The centigrade and réaumur both use the temperature of melting ice as their zero point, while the fahrenheit denominates that temperature as 32 degrees above zero. The fahrenheit zero was supposed to be the lowest temperature attainable with a mixture of ice and salt. The centigrade sets \, 100° as the boiling point of water, and the réaumur \, 80° . The same temperature happens to be represented by \, 212° on the fahrenheit scale. Fahrenheit’s original upper point of reference was not that temperature, but was instead the temperature of the human body. He called this (89:6)
… the \, 96 th degree, and the alcohol expands to that point if the thermometer be held in the mouth or armpit of a healthy person.
It is a great pity that a scale established so awkwardly and so inaccurately should be the one uniformly used in English-speaking countries.
The Establishment of “Fixed Points” (page 252)
The point of departure in the establishment of any thermometer scale is a pair of fixed points, temperatures which can be realized with maximum precision without reference to any thermometer whatever. By common practice, these are now the freezing and boiling points of water at “normal” atmospheric pressure. Huygens is said to have been the first to suggest (in 1665) that thermometers should adopt the boiling temperature of water as a fixed point, and Hooke suggested the same year that its freezing point should be so used. Strangely, it did not seem to occur to either of these doughty pioneers that two fixed points were required to establish a thermometric scale, not merely one. When Celsius established the centigrade scale in 1742 by dividing the temperature range between the freezing and boiling points of water into \, 100 \, equal parts, he chose the boiling point as his zero and measured down to the freezing point, which he called \, 100° . This was soon inverted by a contemporary of his, however, producing the centigrade scale as we know it now (58:118).
The use of expansion as a measure of change of temperature has its limits, of course. It becomes inapplicable both at low temperatures where thermometric substances freeze and at high temperatures where they vaporize. Other methods, electrical and optical, take the place of expansion in these temperature ranges. The instruments used in that connection are too technical to justify description here.
The Effect of Temperature on Gases (page 253)
The fact that it is possible to utilize expansion to indicate temperature implies a certain regularity in such expansion, whether the thermometric substance be a gas, as in Galileo’s thermoscope, or a liquid, as in the later instruments. It is one thing, however, to utilize a natural phenomenon for a particular purpose and quite another to recognize the significance of that phenomenon in anything like its full generality. This has already been pointed out (page 5) as the distinction between mere technology and science. So in the case of thermal expansion, the phenomenon was utilized by all the numerous individuals who invented various kinds of thermometers and constructed temperature scales, but the general laws were discovered and formulated by others.
The behavior of gases under changes of temperature happens to be much simpler than that of liquids and solids, and it is fortunate that this happened to be the first case studied. The earliest study in this field, made by Amontons (1663-1705) in 1699 (165:129), concluded
“that unequal masses of air under equal weights increase equally the force of their spring for equal degrees of heat.”
The context indicates that by “increase in force of spring” the writer means ratio of change in pressure to the original pressure so that the somewhat hazy seventeenth-century phraseology means that air always shows the same value of this ratio for a given temperature change. If this temperature change is specified as \, 1° C. and the lower of the two pressures is that observed when the temperature is \, 0° C., there emerges what is now termed the pressure coefficient. If the higher pressure is \, P_2 \, at \, t_2 °C., the lower pressure is \, P_1 \, at \, t_1 °C., and the pressure is \, P_0 \, at \, 0° C., then, by definition, the pressure coefficient, \, \beta \, , is
\, \beta = \dfrac { P_2 - P_1 } { P_0 (t_2 - t_1) }. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (1)
This notation and terminology was, of course, not involved in Amontons’ experiments, but the basic idea was there, and it was the first time that the idea had appeared anywhere.
The Value of the Pressure Coefficient (page 254)
Evidently the value of the pressure coefficient \, \beta \, can be determined by measuring the pressures of air at each of two observed temperatures while maintaining the volume at a constant value. This [value] comes out to be approximately \, \frac { 1 } { 273 } , a value which has been found to apply, not only to air, but with considerable fidelity to all “permanent” gases, that is, gases which liquify at such low temperatures that their liquefaction was considered impossible until fairly recently. For such gases the value of \, \beta \, is also quite constant over a wide range of temperature. At one time hydrogen was supposed to perform more consistently in this respect than any other gas. For this reason it has been officially designated as the standard thermometric substance. All thermometers are accordingly standardized ultimately by a hydrogen thermometer in which the volume of the gas is held constant, the change of pressure furnishing the measure of change of temperature.
The Concept of “Absolute Zero” (page 254)
Equation (1) may be modified into the following form. Let the upper temperature be \, t \, instead of \, t_2 , and the lower \, 0° C. instead of \, t_1 . Then \, P_1 \, becomes identical with \, P_0 . Making these substitutions and solving for \, P_t \,
\, P_t = P_0 (1+\beta t). \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (2)
Since \, \beta \, has the value \, \frac { 1 } { 273 } , equation (2) states that the pressure of a gas is augmented by \, \frac { 1 } { 273 } rd of its zero-degree value of every degree centigrade that the temperature rises and is diminished by the same measure for every degree that the temperature falls. Thus at \, 273° above zero the pressure of a gas should have double the zero-degree value and at \, 273° below zero the pressure should disappear entirely. This latter hypothetical state of affairs has given rise to the concept of absolute zero, which is a useful concept as long as one does not demand too much from it. So-called absolute temperature (°K) then is expressible by adding \, 273° to the centigrade temperature. Thus \, 0° C. is \, 273° K, and \, 100° C. is \, 373°K, and, of course, \, -273° C. is \, 0° K.
It may not be difficult to imagine the pressure of a gas dropping to zero, but after this has occurred, the substance can scarcely be termed a gas. Moreover, the fact that gases behave in the ordinary range of temperature as though their pressure would become zero at some temperature outside of that range does not necessitate the conclusion that this would actually occur. Amontons, who was the one to get this idea first (in 1703), could be excused for drawing unwarranted conclusions, but it is now known that at low temperatures the value of the pressure coefficient \, \beta \, departs from its conventional value \, \frac { 1 } { 273 } \, for even the most “permanent” of the gases. Nevertheless the concept of absolute zero persists. Its theoretical value is \, -273.18° C., and the lowest temperature actually reached up to the present is within \, .005° of that point.
The Expansivity of Gases (page 255)
If, instead of holding the volume of a gas constant and allowing the pressure to increase as the temperature rises, we reverse the procedure, the proportional change in volume that occurs per degree change in temperature could logically be termed the volume coefficient, as distinguished from the pressure coefficient previously discussed. The term expansivity is considered preferable, however, since it is more general, being applicable to liquids and solids as well as gases. In terms parallel to those defining the pressure coefficient – equation (1) – expansivity of a gas may now be defined as follows:
If the smaller of the two volumes, \, V_1 , is that observed when the temperature is \, t_1 °C. and the higher is \, V_2 \, at \, t_2 °C., that at \, 0° C. being \, V_0 , then the mean expansivity \, \alpha \, is
\, \alpha = \dfrac { V_2 - V_1 } { V_0 (t_2 - t_1) }. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (3)
Corresponding to equation (2) with the analogous notation,
\, V_t = V_0 (1+\alpha t). \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (4)
The first reliable measurements of expansivity of gases were made nearly a century after pressure coefficients had been identified and measured. They were made by Jacques A. C. Charles (1746-1823) about 1787 but were never published. Fifteen years later Joseph L. Gay-Lussac (1788-1850) performed the same experiments with better technique and results (218:27 ff.). He concluded (218:47)
“that all gases, speaking generally, expand to the same extent through equal ranges of heat; provided all are subject to the same conditions.”
This is analogous to the fact that the pressure coefficient is also substantially the same for all permanent gases. The question arises whether the two coefficients are also equal to each other. Gay-Lussac’s value for \, \alpha \, was \, .00375 \, (218:44). This value was refined by later observers to \, .00366 , which is precisely \, \frac { 1 } { 273 } , thus answering the above question in the affirmative.
The Law of Boyle and Gay-Lussac (page 256)
When Boyle’s law was considered in Chapter 18, it was noted that the law was applicable only if the temperature of the expanding or contracting gas was held constant. Now that the response of gas to changes in temperature is known, this limitation may be removed. If we imagine a body of gas undergoing successively a change of temperature (either at constant volume or constant pressure) and a Boyle’s law change of pressure and volume, it may easily be shown that either
\;\;\;\;\;\;\; PV = P_0 V_0 (1 + \beta t) \, (temperature change at constant volume)
or \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (5)
\;\;\;\;\;\;\; PV = P_0 V_0 (1 + \alpha t) \, (temperature change at constant pressure),
where \, t \, is the change of temperature in degrees centigrade, \, P_0 \, and \, V_0 \, are the initial pressure and volume (the initial temperature being supposed \, 0° C.), and \, P \, and \, V \, the final pressure and volume. This is sometimes termed the law of Boyle and Gay-Lussac It might with equal or even greater appropriateness be termed the law of Boyle and Amontons (58:114). It is sometimes put in the form
\;\;\;\;\;\;\; PV = RT \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (6)
where \, T \, is the temperature on the absolute scale and \, R \, is \, \frac {P_0 V_0} {273} .
Equation (6) may be readily deduced from equation (5) by giving \, \alpha \, or \, \beta \, its value \, \frac {1} {273} \, and then changing the temperature scale.
The Expansivity of Liquids (page 257)
Equations (3) and (4) cover the case of the expansivity of liquids as well as gases, except that the value of \, \alpha \, is, in general, very much less than that of gases, is different for different liquids, and bears only approximately the same value in notably different temperature ranges even for the same liquid. The pressure coefficient \, \beta \, – equation (1) – has no practical significance in connection with liquids. Liquids are so incompressible that no ordinary container could withstand the pressures necessary to maintain the volume unchanged as temperature rises.
The idea of measuring the expansivity of a liquid seems first to have occurred in 1723 to Brook Taylor, the author of the famous mathematical theorem that bears his name. Taylor undertook to find out whether linseed oil, which at that time was frequently used in thermometers as we use mercury now, really expanded proportionally to increases of temperature. In describing his experiment he said (276:190):
“I successively filled the Vessel (in which the linseed-oil thermometer, graduated in volumes rather than in degrees, was to be immersed) with one, two, three, etc., Parts of hot boiling water; and in every case I immersed the Thermometer into the water and observed to what Mark it rose…. And having observed where the Thermometer stood in cold water, I found that its rising from the Mark, or the Expansion of the Oil, was accurately proportional to the Quantity of hot water in the Mixture, that is, to the Degree of Heat.”
The increase in volume of a liquid with rising temperature, which Taylor found to be measurably proportional to the rise in temperature, is accompanied, of course, by a decrease in its density. Advantage of this is taken in hot-water heating systems, which depend for their action upon the expansivity of water. So-called convection currents are set up by the sinking of the more dense cooled water in the upper portions of heating systems into the “boilers” below, thus continuously exchanging places with the water previously heated. Hot-air furnaces work on the same principle, except when circulation is effected by motor-driven fans. The draft in chimneys is similarly induced, and most meteorological phenomena, including winds, cyclones, tornadoes, and cloud formation, are principally large-scale manifestations of convection currents set up by localized expansions and contractions of air.
The Anomalous Expansivity of Water (page 257)
Though all liquids expand non-uniformly, water, the most common liquid, shows this in a particularly flagrant manner. This is indicated graphically in Fig. 139. This shows, with temperatures as abscissas and volumes as ordinates, the volume of a mass of water which occupies unit volume at \, 4° C. The anomalous behavior of water consists in the fact that the volume is a minimum at this temperature, increasing for lower as well as for higher temperatures. This means that the value of \, \alpha \, is negative for temperatures below \, 4° C., zero at that temperature, and positive for all higher temperatures. Fig. 139 may be compared with Fig. 138, in which the uniformity of the expansivity of air, indicated by the straightness of the line in the earlier figure, is in contrast with the non-uniformity of the expansivity of water as shown in the later figure. In both cases the expansivity is proportional to the slope of the curve. The expansivity of water at different temperatures may be estimated from the slope of the curve in Fig. 139 and will be seen to vary from approximately \, -.0001 \, at \,-2° C., to \, .0001 \, at \, +12° C.
That ice forms on top of bodies of water instead of below the surface is due to this peculiarity. Were it not for the negative value of the expansivity of water just above the freezing temperature, many forms of aquatic life now existing would never have come into being.
[…]
A Logical Difficulty (page 260)
In the foregoing chapter, there may be found a good example of the evolution of scientific concepts in the development of the idea of temperature. The unsatisfactory nature of mere sensation as a means of specifying temperature is well known. The adoption of expansion as a more observable effect of temperature, however, raises a logical difficulty. What right have we to say that when a fixed point for \, 0° C. and \, 100° C. have been established on, say, a mercury thermometer, the \, 50° C. reading indicates a temperature halfway between the two extremes? If we say so simply by definition, our confidence in the validity of the definition is shaken by the discovery that if the thermometric substance had been alcohol or linseed oil – both have been used in the same rôle as mercury – the mid-reading on the thermometer would have occurred at a different temperature. What logic is there in giving mercury the place of precedence as a thermometric substance?
This difficulty, occasioned by non-uniformity in the expansivity of liquids, has led in practice to the designation of a gas (hydrogen) as the standard thermometric substance. This reduces the practical difficulty, since gases expand more nearly in agreement with one another than do liquids or solids. It does not, however, remove the logical tour de force involved in equating volume or pressure changes to temperature changes.
Brook Taylor (page 257) removed the logical difficulty by taking temperature levels of mixtures of hot and cold water as proportional to the relative amounts of boiling water and cold water in the mixtures. But the method is too inaccurate to be practicable, in spite of its logical correctness. A theoretical criterion, devised by Lord Kelvin, comparable to Brook Taylor’s laboratory scheme, will be established in a later chapter. In the meantime we shall have to content ourselves, as our scientific predecessors did, with tentative standards of temperature which involve a somewhat disquieting appearance of being arbitrary.
Analogical logical difficulties appear elsewhere in physics, as in equating pitch to frequency and loudness to energy in sound, difficulties which create some very real practical problems. But if the scientific era has taught us anything, it is that we must define our concepts in terms of operations even if logical symmetry is thereby sacrificed. Not since the days of the Scholastics has it been good intellectual form to give logic precedence over experiment.
/////// End of Quote from Taylor
/////// Quoting Taylor (Volume 1, p. 264)
The Idea of Heat as a Substance (page 264)
It has been said (249:22) that “it is difficult for the human mind to think at all unless it has something to think about.”
This is simply another way of saying that it is easier to think in concrete than in abstract terms. That the scientific treatment of mechanics was well advanced long before heat yielded itself to the same approach is doubtless partly because the former dealt more with objects and palpable processes than did the latter. To make progress in the study of heat it was therefore natural that men should have conjured up more or less spontaneously a hypothetical entity whose sole function it was to constitute the “substance” to which the term could refer.
The concept of temperature, developed in the previous chapter, is unquestionably the most fundamental of those involved in the scientific study of heat. If the temperature of an object rises or falls it is natural to imagine that this is because the object receives or loses something termed “heat.” To the modern mind, this might be an outgrowth of observations such as that the rise and fall of pressure in an automobile tire is occasioned by the receipt or the loss of air. The difference in the two cases lies in the fact that air exhibits other attributes than that of keeping tires inflated, whereas the corresponding attributes of heat are not so clear. Nevertheless, all of us, even the toughest-minded physicist, continue to speak of the flow of heat. This not only shows that we still find it convenient to speak of heat in terminology appropriate when heat was considered an actual substance, but also gives a clue as to what kind of substance we are thinking about.
Early Confusion Between Heat and Temperature (page 264)
Though there is a tendency to assume a somewhat patronizing attitude when the old concept of heat as a substance is mentioned, the fact is that precisely this concept first made it possible to treat the subject of heat scientifically. Prior to the middle of the eighteenth century, heat, temperature, and fire all meant much the same thing. A good example of this confusion of ideas is to be found in one of the outstanding treatises on chemistry of the first half of the eighteenth century (Hermann Boerhaave, Elementa Chemiae, 1732). Remarking that heat equilibrium between adjacent bodies consisted of equal temperature among them, the author expressed the opinion that when this condition obtained, there was an equal quantity of heat in every equal volume, whatever bodies occupied those volumes. The reason he gave for this was that a thermometer indicated the same temperature to whichever of those bodies it was applied.
This we know today was a very serious misapprehension. It could have been dispelled, if the ideas and equipment had been available, by comparing the quantity of heat necessary to warm up a body of air with that necessary to warm up the same volume of water between the same temperature levels. The water would have been found to require more than 3000 times as much heat as the air. Hence, if both had been placed in the same enclosure and had reached the same temperature, their respective heat contents would have been utterly different. However, the procedure for measuring quantity of heat, as distinct from temperature, was not known at that time. Indeed, that there was any difference between heat and temperature was not realized. The distinction was first made when the concept of heat as a sort of fluid became clear – temperature becoming merely one of the attributes of that fluid, somewhat as pressure is the attribute of a gas or liquid.
The Distinction Between Heat and Temperature (page 265)
The first man to make a clear distinction between temperature and quantity of heat was Joseph Black (1728-1799) of the Universities of Glasgow and Edinburgh. About the middle of the century he was commenting to his classes on the fallacy in the equal-heat-content theory which prevailed at the time and which was described above. He said in rejoinder (165:135):
This is taking a very hasty view of the subject It is confounding the quantity of heat in different bodies with its general strength or intensity [i.e., temperature] though it is plain that these are two different things, and should always be distinguished, when we are thinking of the distribution of heat.
Black did not coin a name for the fluid which he thought constituted heat. He usually referred to it as the “matter of heat” or, when he was concerned with its amount, as the “quantity of heat,” as in the above quotation. Some time during the latter part of his life the name caloric was coined to denominate the “matter of heat,” and Black’s views on the nature of heat have accordingly come to be known as the “caloric” theory. Black himself never used the term, however.
Early Ideas on the Nature of Heat (page 266)
As the concept of caloric became more explicit, it was natural that attempts be made to learn about other physical properties of heat besides its ability to influence temperature – especially its weight. At first the phenomena of combustion seemed to furnish evidence on this point. Combustible materials were known to give off heat in the process of burning and lose weight. Moreover, certain metals when heated were reduced to powder, and the weight of the powder had been found to be greater than the weight of the original metal. This seemed to point in the same direction, since it was imagined that the heat from a burning flame entered a metal which was held in it and increased the weight of the metal. Robert Boyle became very insistent, long before Black’s day, on the significance of this phenomenon (Science Progress, 29, 253 (1934)).
A number of experimenters weighed the same object first when it was hot and then when it was cold in order to detect differences in weight. The results were conflicting and indicated more often than not that the object weighed more when it was cold than when it was heated (163:137-48). But the final result, secured by Count Rumford, who played a very prominent part on the later developments in heat, led to his conclusion that if, as his measurements clearly indicated,
“the weight of gold is neither augmented nor lessened by one millionth part, upon being heated from the point of freezing water to that of a bright red heat, I think we may safely conclude that all attempts to discover any effect of heat upon the apparent weights of bodies will be fruitless.”
This did not discourage the calorists, however. It simply made it necessary to postulate that caloric was weightless. This did not appear as untenable an hypothesis in the eighteenth century as it does now, for weightless fluids had been invoked for centuries to “explain” a variety of phenomena and were still intellectually respectable. Electricity was such a fluid, magnetism another, and light, if not a fluid, was overwhelmingly conceded at that time to be a weightless corpuscular substance. In addition a very old idea, that of the “ether,” while temporarily in eclipse during the eighteenth century, became exceedingly fruitful during the nineteenth.
Birth of the Concept of Specific Heat (page 267)
The observation which Black had made – to the effect that the distribution of heat between objects at the same temperature was not at all in proportion to their volumes – raised questions as to how it was distributed. Another theory was that it was in proportion to their weights. This, too, Black showed to be incorrect. He referred to some experiments by Fahrenheit in which water and mercury at different temperatures were mixed. The heat yielded to the mixture was found not to be proportional either to the volume or to the weight of whichever was the hotter of the two liquids. He stated his conclusion thus (165:136-39):
“This shews that the same quantity of the matter of heat has more effect in heating quicksilver than in heating an equal measure of water, and therefore that a smaller quantity of it is sufficient for increasing the sensible heat of quicksilver by the same number of degrees…. Quicksilver, therefore, has less capacity for the matter of heat than water has; it requires a smaller quantity of it to raise its temperature by the same number of degrees.
We must therefore conclude that different bodies, although they be of the same size, or even of the same weight, when they are reduced to the same temperature or degree of heat, whatever that may be, may contain very different quantities of the matter of heat; which different quantities are necessary to bring them to this level, or equilibrium with one another.”
This idea of Black’s of a certain “capacity for heat” characteristic of each substance was new and proved to be correct. His terminology, however, was soon modified, the term specific heat being introduced by some of his contemporaries to describe essentially what Black meant by “capacity for heat” (276:183, 204).
Definition of Specific Heat (page 268)
In Chapter 8, the convenience of having some standard of reference for densities was pointed out. The density of water being accepted in this rôle, the ratio of the density of any given substance to that of water assumed some importance and was termed the specific gravity of the substance in question. Water has also come to be the standard for specific heats, the specific heat of any substance being defined as the ratio of the amount of heat required to warm a given mass of the substance between two temperatures to the amount similarly required for water in a particular temperature range.
The method of measuring specific heats is described as follows in the Notes added to Black’s Lectures by his editor (163:124).
Assume that a certain mass of the substance under investigation has been heated and then immersed in a measured mass of cold water, heat losses to containers and their surroundings being discarded. (These corrections, along with several others, must in practice be made.) Then
multiply the weight of the water by its change of temperature. Do the same for the other substance. Divide the first product by the second. The quotient is the capacity [specific heat] of the other substance, that of water being accounted unity.
The agreement of this method of measurement with the definition of specific heat may readily be shown. Thus the ratio defined in that definition may be stated in the form of the following compound fraction:
\, \dfrac { \;\; \dfrac { \text {heat lost to the water by the warmer substance} } { \text {mass of substance \, x \, diminuition of temperature} } \;\; } { \;\; \dfrac { \text {heat gained by the water} } { \text {mass of water \, x \, rise of temperature } } \;\; } \,This is precisely Black’s statement on the numerical value of specific heat.
Like specific gravity, which changes its value in consequence of the thermal expansion of the substance, specific heat also depends upon temperature. Tables of values of either entity always specify the temperatures for which the tabular values apply.
The Unity of Quantity of Heat (page 269)
The creation of the concept of quantity of heat necessitated the choice of a unit. The calorists employed as this unit the quantity of heat that must enter a unit mass of water to raise its temperature by one degree; or they reversed the definition to involve the heat that must be abstracted from a unit mass of water to lower its temperature by one degree, experiment having demonstrated that the quantity of heat involved was the same in either case. This definition is still valid. In metric units, the calorie is defined as the quantity of as the quantity of heat transferred whenever one kilogram of water changes its temperature by one degree centigrade. As thus defined the calorie has slightly different values at different temperature levels, since the remark of the preceding paragraph applies to water as well to other substances. The value of the calorie fluctuates between extremes of about a half a percent above and below its mean value. For accurate work a more precise definition is necessary, but it need scarcely be formulated here – partly in view of the fact that there is as yet no complete agreement on what this definition should be.
Ways in Which Heat is Transferred (page 269)
On page 257 one of the ways of transferring heat was mentioned, that of convection currents. The idea is almost absurdly simple, and would scarcely merit further mention were convection not commonly included in a trilogy of ways in which transference of heat is commonly stated to occur. Convection, involving motion of the medium, is more of a mechanical than a heat phenomenon since the actual heating and cooling of the medium can take place in no other way than by conduction or radiation. Convection is, however, one of the major elements in meteorology, since terrestrial air motions of all kinds are simply convection currents on a huge scale.
Conduction has been defined (173:10) as
the flow of heat through an unequally heated body from places of higher to places of lower temperature.
In this process, unlike convection, the heated substance does not migrate. Obviously, conduction can occur when the heated substance is in any one of its three states: solid, liquid, or gaseous, while convection is necessarily confined to the last two.
Radiation, or radiant energy, has been similarly defined (loc. cit.) as a process by which
the hotter body loses heat and the colder body receives heat through some intervening medium which does not itself thereby become hot.
The most impressive example of radiation is the transfer of heat from the sun to the earth through regions of space known to be frigid in the extreme, but the process occurs in greater or less measure wherever differences of temperature exist. It is worthy of note that the entity in transit by radiation cannot properly be called heat until absorbed at the end of its journey whereupon it reassumes its ability to elevate temperature.
Radiation may be compared to a radio broadcast; indeed, radiation actually is a broadcast, though on wavelengths much shorter than those to which a radio receiving set can be tuned. Sounds are converted at a broadcast studio into an electromagnetic disturbance which, whatever it may be, is certainly no longer sound. Captured by a receiving set this disturbance is reconverted into sounds tolerably similar to those which had entered the microphone. In a closely analogous way, the heat of the sun is converted into radiant energy and transmitted into space. Some of it is intercepted by the earth and other astronomical bodies and absorbed. In the process of absorption it is reconverted into heat.
Thermal Conductivity (page 270)
[…]
The Discovery of Radiant Energy (page 273)
The fact that radiation from the sun travels in some other guise than heat was mentioned on page 270. The fact that the paths followed by this radiation can be traced by tracing the accompanying light furnishes a temptation to identify heat radiation with light. In the common use of burning glasses over a period of many centuries no clear distinction was ever made until the seventeenth century between the light and the heat that were focused by the glasses. But in 1620 Francis Bacon showed the first clear recognition of the possibility that the two might be handled separately. He said (23:127):
“Let the burning glass be tried on warm objects which emit no luminous rays, as heated but not ignited [i.e., not incandescent] iron or stone or hot water, or the like; and observe whether the heat becomes increased and condensed, as happens with solar rays.”
About sixty years later Mariotte discovered another phenomenon which emphasized still further the distinction between light and heat radiation. Though the two remained together when sunlight was acted upon by a burning glass, they were separated by a burning glass when the source was a fire instead of the sun. In his experiment he put a concave metal mirror before a fire. At its focus the hand could not long endure the heat; but when a glass plate was placed over the mirror, heat could no longer be felt at the focus, though the light was substantially undiminished (170:f:303,344).
More than a hundred years elapsed before further steps were made toward an understanding of heat radiation. In 1777 Carl Scheele (1742-86) repeated and extended Mariotte’s observations (228:120 ff.) not only distinguishing radiant energy from light on the one hand, as Bacon and Mariotte had done, but also distinguishing it from “fire” or ordinary heat on the other hand. He coined the name “radiant heat” and spoke of “the radiant heat which is invisible and differs from fire,” that is to say, which is neither light nor ordinary heat.
Mariotte’s observation furnishes the explanation for the action of the familiar “hothouse.” Glass transmits radiation from the sun but is opaque to radiation from lower-temperature sources, such as stoves, and even more so to radiation from sources of still lower temperature, such as warm earth. Hence, a glass-covered box exposed to the sun acts as a “heat trap” and permits the enclosed earth to build up temperatures much above those of the surroundings.
Subsequent studies have made it clear that radiation is much more analogous to light than to heat. The relation is, indeed, much closer than that of mere analogy. Because of this fact, it will be of advantage to defer a consideration of some of the attributes of radiation until the characteristics of light have been studied (page 541 ff.). Others lend themselves to treatment without the concepts furnished by a study of light.
The Apparent radiation of Cold (page 274)
As early as the sixteenth century Giambattista della Porta, whose contributions to light at the tender age of fifteen will presently be considered, had remarked (207: 264) that a concave mirror reflected sound, light, heat, and cold. Noting that the light of a candle set before a mirror and its heat each produced its appropriate sensation on the eye placed at the conjugate focus, he remarked
” but this is more wonderful, that, as heat, so cold should be reflected: if you put snow in that place if it comes to the eye … it (the eye) will presently feel the cold.”
A similar effect was identified by subsequent observers. The observation lent some weight for a time to a theory that there was a fluid carrying the attributes of cold just as there had been assumed to be the fluid called “caloric.” But that theory was short-lived. It did, however, produce more substantial fruit in what has become known as Prévost’s theory of exchanges in 1791. The correct interpretation of Porta’s observation was not that the ice radiated “cold” to the eye but that the eye, being the warmer of the two, radiated heat to the ice and felt cold in consequence.
Prévost’s Theory of Exchanges (page 274)
Prévost asked himself in effect whether the warm eye ceased radiating whenever a warmer body was substituted for the ice. He concluded that it did not; a warmer body was substituted for the ice. He concluded that it did not; that, on the contrary, every body radiated heat no matter what its temperature was; but that warmer bodies radiated more rapidly than cooler and that, hence, the net flow was from the warmer to cooler bodies. He considered that the ultimate state of equal temperature represented not the cessation of radiation, but merely a state of dynamic equilibrium in which the bodies radiated and absorbed heat at equal rates.
This point of view clears up some phenomena that are often sources of perplexity. One such is the unreliability of a thermometer exposed to the sun. The usual requirement of a thermometer is that it shall indicate the temperature of the surrounding air – which it has an opportunity to do in the shade. But if exposed to the sun, it absorbs radiation from the sun in addition to heat received by conduction from the surrounding air, converts the absorbed radiation into heat, and, hence, registers a temperature above that of its surroundings.
The amount of this excess depends on the thermometer. It would be small if the bulb contained alcohol, since much of the radiation would simply travel through the transparent liquid instead of being absorbed by it. The excess would be greater for opaque mercury, but the silver surface would reflect a great deal of the radiation and thus also fail to register its full effect. But if the bulb were painted a dead black, most of the radiation incident on it would be absorbed, and the thermometer would register a temperature far higher than that of its surroundings.
It thus becomes clear that it is entirely meaningless to inquire about the temperature in the sunshine. Every object exposed to the sun assumes a different temperature, the theoretical upper limit being the temperature of the sun, for naturally if it should exceed that temperature, an object would radiate more heat to the sun than it receives from it.
Laws of Cooling (page 275)
Having observed that rates of cooling of warm bodies were greater the higher their temperatures above the surroundings, Newton made a statement in 1701 involving the hypothesis that the rate of cooling is actually proportional to excess of temperature over that of the surroundings. This is commonly called Newton’s law of cooling. It was never intended as a law of radiation, though it is sometimes classified as such and criticized in consequence for its inaccuracy. It was merely an attempt to describe, on a purely empirical basis, the rate of cooling of hot specimens ordinarily encountered. While this cooling is due in part to radiation, conduction to the surrounding air also makes a contribution. This contribution is, of course, influenced by the convection currents thereby set up. Within its province Newton’s law of cooling is a useful approximation applying to small differences of temperature, but it is nothing more.
Almost two centuries after Newton’s time (1870) the real law of cooling by radiation was discovered by Joseph Stefan (1835-93). It is usually termed Stefan’s fourth power law. He said (165:378):
“We obtain numbers which come very close to the [observed] rates of cooling if we assume that the heat radiated by a body is proportional to the fourth power of its absolute temperature.”
Stefan was thinking of radiation in the same sense that Prévost had been thinking of it nearly a hundred years earlier. Thus the net radiation between a heated object and its surroundings would be proportional to the difference of the fourth powers of the respective absolute temperatures.
Compare, for example, the radiation from a piece of metal at the temperature of boiling water ( 100° C.) with its radiation when heated to the point where it barely glows in the dark, say \, 500° C., the surroundings being at room temperature, say \, 20° C. The ratio would be
\, \dfrac { (273+500)^4 - (273+20)^4 } { (273+100)^4 - (273+20)^4 } = \dfrac { (35.7 - 0.74) \times {10}^{10} } { (1.93 - 0.74) \times {10}^{10} } = 29.4. \,Thus a piece of iron barely red hot radiates heat about thirty times as fast as it does when at the temperature of boiling water. Though it is quite unfair to Newton’s law to invoke it in such a case, the corresponding calculation is \, \frac {500-20} {100-20} = 6 , a notable discrepancy.
It is interesting to observe, however, that if the two temperatures are taken at \, 22° C. and \, 21° C., the room temperature being still \, 20° C., the two results agree within a fraction of one per cent.
Speculations on the Nature of Heat (page 276)
During the whole period covered by this account, the nature of heat had been a controversial question. Black, as has been seen, was inclined to the opinion that “heat was the effect of a peculiar substance” (37:49), but he was very cautious about committing himself too far. He shared Newton’s wariness of hypothesis (compare 190:113). He expressed the opinion once (37:192-94) that all speculations were hypothetical and to be avoided
“as taking up time which may be better employed in learning more of the general laws of chemical operations.”
The doubt about the nature of heat continued until the middle of the nineteenth century. There were two main schools of thought, those now called the calorists, who considered heat to be a weightless fluid of some sort, and those who had a more or less accurate prevision of the modern view, that heat was of the nature of molecular agitation. The calorists accounted for conduction by attributing a self-repellent property to caloric along with an attraction for caloric on the part of matter. They were saved from the complicated task of trying to reconcile the caloric theory with the facts of radiation by the lateness with which those facts came to common knowledge.
The Genesis of the Kinetic Hypothesis of Heat (page 277)
The idea, to use the title of Tyndall‘s 1863 book of lectures, of Heat Considered as a Mode of Motion is of much longer standing than is ordinarily realized. Disregarding some Greek speculations, one can find that early in the scientific era, 1706, John Locke said
“Heat is a very brisk agitation of the insensible parts of the object, which produces in us the sensation from whence we denominate the object hot, so what in our sensation is heat, in the object is nothing but motion.”
In 1738, Daniel Bernoulli had remarked (165:250) that
“it is admitted that heat may be considered as an increasing internal motion of the particles.”
In 1780, Lavoisier and Laplace were even more explicit. They said in their Mémoire sur La Chaleur (201:58:
“…heat is the vis viva resulting from the insensible movements of the molecules of a body. It is the sum of the products of the mass of each molecule by the square of its velocity.”
In 1798, Count Rumford, drawing conclusions from an experiment which has become one of the classics of science and which will be examined at length in Chapter 22, stated that it appeared to him to be
“extremely difficult if not quite impossible to form any distinct idea of anything, capable of being exited and communicated, in the manner the Heat was exited and communicated in these Experiments, except it be MOTION.”
Most of these pronouncements, with the exception of Rumford’s, went considerably beyond any really justifiable evidence from experimental data. But subsequent developments were destined to vindicate these early opinions, nevertheless. The nature of some of this evidence will appear in the succeeding two chapters.
Change of State
The Beginning of a New Idea (page 279)
Black avoided a commitment to any theory of the nature of heat in so far as it was possible. As long as he was dealing merely with the flow of heat as associated with changes of temperature, this avoidance of a troublesome problem was feasible within certain limits. But it ceased to be so easy when he turned his attention to another class of phenomena, changes of state.
But Black’s work was not confined to setting up the concept of specific heat and laying the foundation for the definition of a heat unit. These marked the beginning of the science of heat by making the first and all-important distinction between temperature and quantity of heat. Black naturally turned next to the heat relations involved in transformations between solid, liquid, and gaseous states of aggregation.
These needed clarification. Black pointed out that even a casual consideration of the prevailing ideas on melting and freezing, for example, showed that they must be erroneous. He remarked (37:116 ff.):
“Fluidity was universally considered as produced by a small addition to the quantity of heat which a body contains when it is once heated up to the melting point…. If this common opinion had been well founded, if the complete change (of ice or snow) into water required only the further addition of a very small quantity of heat, the mass, though of considerable size, ought all to be melted is a very few minutes or seconds, the heat continuing incessantly to be communicated from the air around….
This sudden liquefaction does not actually happen. The masses of ice or snow, after they begin to melt, often require many weeks of warm weather, before they are totally dissolved into water….
This remarkable slowness struck me as quite inconsistent with the common opinion of the modification of heat in the liquefaction of bodies…. It is therefore evident that the melting ice receives heat very fast, but the only effect of this heat is to change it into water. A thermometer, applied to the drops or small streams of water, immediately as it comes from the melting ice, will point to the same degree as when applied to the ice itself. A great quantity, therefore, of the matter of heat which enters into the melting ice, produces no effect but to give it fluidity, without augmenting its sensible heat; it appears to be absorbed and concealed within the water, so as not to be discoverable by the application of a thermometer.
In order to understand this absorption of heat into the melting ice and concealment of it in the water more distinctly, I made (among others) the following experiment…. I put a lump of ice into an equal quantity of water heated to the temperature of \, 80° C. and the result was that when the ice was all melted the fluid was no hotter than water just ready to freeze. Nay, if a little sea salt be added to the water and it be heated only to \, 74° \, or \, 76° , we shall produce a fluid sensibly colder than the ice was in the beginning, which has appeared a curious and puzzling thing to those unacquainted with the general fact….
I shall now mention another example, an experiment first made by Fahrenheit [Philosophical Transactions, 33, 78 (1724)]. He … exposed globes of water to frosty weather so long that he had reason to be satisfied that they were cooled down to the degree of the air, which was four or five degrees below the freezing point. The water, however, still remained fluid, so long as the glasses were left undisturbed, but, on being taken up and shaken a little a sudden freezing of a part of the water was instantly seen…. But the most remarkable fact is, that while this happens the mixture of ice and water suddenly becomes warmer, and makes a thermometer, immersed in it, rise to the freezing point.”
The Heat of Fusion (page 280)
Only in a field in which scientific development was long overdue could almost casual observations have been so devastating to previous views. What would seem to be the inescapable implication in the length of time required for ice to melt even in warm weather had been lost on previous observers. It would seem as though anybody might have observed that a chunk of ice would cool an equal weight of water through \, 80° C. and have drawn a correct deduction therefrom. And even as acute an experimenter as Fahrenheit had failed to grasp the significance of his sub-cooling and freezing experiment of forty years before. These observations pointed unequivocally toward the absorption of a huge quantity of heat in the process of melting, unaccompanied by any rise in temperature, and the evolution of a corresponding quantity in freezing, a concept not theretofore envisioned by anyone.
Black coined the term “latent heat” to describe the heat which seemed thus to become concealed when fusion occurred and mysteriously reappear when freezing set in. The term, while still in use, is giving way to the more descriptive term heat of fusion. One may conclude correctly from Black’s observations that the heat of fusion of ice is 80 calories per gram.
Black had remarked on the restraining effect which “latent heat” exerted on spring floods. Referring to the prevailing opinion as to the negligible quantity of heat required merely to melt ice and snow, he said:
“Were this really the case, the consequence of it would be dreadful in many cases; for, even as things are at present, the melting of great quantities of snow and ice occasions violent torrents, and great inundations in the cold countries, or in the rivers that come from them. But were the ice and snow to melt as suddenly as they must necessarily do, were the former opinion of the action of heat in melting them well founded, the torrents and inundations would be incomparably more irresistible and dreadful. They would tear up and sweep away everything, and that so suddenly that mankind should have great difficulty to escape from their ravages.”
Black failed to mention another respect in which the heat of fusion affected climate, namely, the way that it served to reduce temperature fluctuations near large bodies of water in winter. Today both the deferment of rises in temperature, because spring heat is absorbed in the melting of ice, and the tempering of the lowest winter temperatures, because heat is evolved by the freezing of water, are matters of common knowledge. Farmers sometimes protect stored fruit from freezing by providing large tubs of water; the freezing of the water at a temperature slightly above that which would damage the fruits evolves heat which prevents the room from becoming colder as long as any considerable amount of water remains unfrozen.
The Influence of Pressure on the Freezing Point (page 281)
[…]
Heat and Mechanical Energy
Science and Technology (page 291)
The next step toward a comprehension of the nature of heat was in part an outgrowth of the engineering developments of the eighteenth century. It constitutes an illustration of the continual interplay between the physical sciences and technology. Technology consists primarily in the adaptation of scientific principles to utilitarian ends. Its motivation is filling the requirements of daily life in a machine age, and its working materials are the discovered laws of science. Without the basic sciences it could not exist. On the other hand, the sciences frequently find in technology fertile fields for exploration. This is because technology incorporates much of the unanalyzed accumulated experience of the race along with the scientific materials which it utilizes. The unrecognized mysteries of the commonplace constitute an inexhaustible store of scientific problems. One needs only to consider Archimedes and the balance, Galileo and the falling bodies, Newton and the apple, Pascal and the water wheel, and Black and the flow of heat, to appreciate this fact.
On of the early contributors to our knowledge of the nature of heat commented on the storehouse of material that science might find in technology. Count Rumford, about whom much will soon be said, remarked in 1798:
“It frequently happens that, in the ordinary affairs and occupations of life, opportunities present themselves of contemplating some of the most curious operations of nature, and very interesting philosophical experiments might often be made, almost without trouble or expense, by means of machinery contrived for the mere mechanical purposes of the arts and manufactures.
I have frequently had occasion to make this observation; and am persuaded, that a habit of keeping the eyes open to everything that is going on in the ordinary course of business life has oftener led, as it were by accident, or in the playful excursions of the imagination, put into action by contemplating the most common appearances, to useful doubts and sensible schemes for investigation and improvement, than all the more intense meditations of philosophers, in the hours expressly set apart for study.”
The substance of the present chapter constitutes an eloquent example of this observation of Rumford.
Early Steam Engines (page 292)
In 1763 James Watt (1736-1819), scientific instrument maker in the University of Glasgow, was asked to repair a model of a type of “fire engine,” as steam engines were called in those days, which had been in use for about fifty years. Several hundred of them were then pumping water from deep mines in England and on the European continent. It was a very crude affair indeed, and apparently Watt considered it so, for he set about devising the improvements which have made his name immortal.
Nowadays the idea of such an engine involves the action of steam pressure on a piston working through a crank to rotate a flywheel. There was probably no flywheel on this engine, for though the crank had been patented more than a quarter of a century earlier, the so-called “Newcomen” engine which Watt was repairing did not incorporate it. Moreover, though it was really a steam engine, it was not, paradoxically, actuated by steam pressure. It was instead driven by atmospheric pressure. The only function of the steam was to produce a vacuum by its condensation on one side of the piston to give atmospheric pressure a chance to push on the other side. All the steam in the boiler was consumed at each stroke, and sufficient time had to elapse between strokes to allow the accumulation of enough steam to proceed. The operation of the Newcomen engine must have been awe-inspiring if size, vibration, and noise would make it so. One of the perennial problems of the steam engineer of that day was that when the cylinder was mounted above the boiler, as in Fig. 150, the brickwork was continually crumbling under the pounding of the engine. If it was mounted at one side, then the joints in the steam pipe required constant repair (286:615). It was no wonder that Watt could see ways of improving the Newcomen engine.
The Significance of the Steam Engine (page 292)
But the important thing was, not that the engine was so crude, nor even that it started Watt in the construction of a better one, but that it was a practicable device for converting heat into mechanical energy and utilizing it as a source of power. The discovery that heat could be so converted possessed enormous potentialities, not merely in unlocking the sources of power that were to create the Industrial Revolution, but also in giving emphasis to a phenomenon which was destined to shed much light on the nature of heat itself.
But a typical example of human inability to distinguish what was later seen to be the immediate implications of a discovery is the fact that the bearing of the steam engine on the nature of heat was as little foreseen at this time as was its contribution to the Industrial Revolution. And this was true in spite of the fact that Watt collaborated closely with Black, the outstanding contemporary authority on heat, in Watt’s studies incident to the improvements which he made on the steam engine.
More than sixty years were to elapse before the general realization dawned in scientific circles that the steam engine was merely a special device for converting heat into mechanical energy; and eighty, before the obvious deduction was drawn that heat and mechanical energy were interconvertible and possessed measurable proportionality to each other.
Count Rumford Enters the Scene (page 293)
But, through a surprising obtuseness seemed to delay the recognition that heat could be converted into mechanical energy, a somewhat greater degree of discernment attended the early observations of the conversion of mechanical energy into heat. The first of these observations brings into the scene one BENJAMIN RHOMPSON (1753-1814) born in North Woburn, Massachusetts, a picturesque character who became an officer in the British army during the American Revolution and later entered the service of the Elector of Bavaria. The Elector, in recognition of notable achievements, made him a Count. Thompson helped to found the Royal Institution in London and throughout his entire life was a singularly able man of science. He is better known as Count Rumford. Rumford was the original name of Concord, New Hampshire, where he owned an estate. Late in life he married the widow of the famous chemist Lavoisier, who had been guillotined during the french Revolution. The marriage was not a happy one, and Rumford somewhat ungallantly implied later that Lavoisier had been fortunate in his fate.
While supervising the boring of a brass cannon for the Bavarian army, Rumford’s attention was arrested by the amount of heat generated by friction of the boring tool with the gun. It seemed so disproportionately large to come out of the objects evolving it that Rumford’s curiosity was piqued as to the source of such large amounts of heat. The caloric theory attributed heat produced in this way to the squeezing out of caloric from the brass by the action of the boring machinery. This explanation apparently did not appeal to Rumford. It occurred to him that if this were correct, there should be less caloric in the shavings than in the same weight of solid brass, because so much would have evolved in the process of converting the brass to shavings. Rumford decided to find out whether the shavings gave out less heat in the process of cooling than did the same weight of solid brass. That is, he compared the specific heats of the brass in the two states, but could find no difference between them. He said (165:152):
“But no such change had taken place; for I found, upon taking equal quantities, by weight, of these chips and of slips of the same block of metal … that the portion of water into which the hot chips were put was not, to all appearance, heated either less or more than the other portion, in which the slips of equally hot metal were put….
From hence it is evident, that the Heat produced could not possibly have been furnished at the expense of the latent heat [i.e., the specific heat] of the metallic chips.
What Was the Origin of Frictional Heat? (page 294)
The discovery that the frictional heat had not been abraded out of the metal left unanswered the original question as to where it did originate. Rumford’s suspicion fell upon the horse which, by a treadmill, was furnishing the power for the boring operations. (In spite of Watt’s improvements, the steam engine had apparently not come into common use, at least on the continent, as late as 1798.) He was impressed (165:160) with
“how large a quantity of Heat might be produced, by proper mechanical contrivance, merely by the strength of a horse, without either fire, light, combustion or chemical decomposition; and in a case of necessity, the Heat thus produced might be used in cooking victuals.
But no circumstances can be imagined in which this method of procuring Heat would not be disadvantageous; for more Heat might be obtained by using the fodder necessary, for the support of the horse, as fuel.”
One is tempted to speculate whether Rumford may not, in this surmise, have caught a fleeting vision of the idea that the heat evolve by his boring mechanism was actually a part of the heat or combustion of the fodder which the horse had eaten. A few years before this Lavoisier and Laplace had noted that animal heat was produced by a guinea pig at about the same rate as would be evolved by the steady burning of the food which it had eaten (69:151), a verification and refinement of an idea which Mayow had proposed a century before (70:133).
Close as Rumford came to this major inspiration, he seems to have missed it. But he caught another which anticipated future developments even more clearly. He had noticed that the quantity of heat developed in the process of boring was not at all proportional to the amount of metal removed. By using a blunt borer he could, in fact, develop an indefinite quantity of heat without the removal of any appreciable amount of metal. The heat developed seemed rather to be proportional to the work done by the horse, whether much or little metal was removed by the process. So he set about the measurement of the constant of proportionality, specifically the number of foot-pounds of work necessary to heat one pound of water \, 1° F. (This latter is the British thermal unit (B.T.U), analogous to the calorie of the metric system.)
First Determination of the Mechanical Equivalent of Heat (page 295)
Rumford observed that
“the total quantity of ice-cold water with which the heat actually generated by friction and accumulated in \, 2 \frac {1}{2} \, hours was \, 150 \times 33,000 \, foot-pounds. Hence, the number of foot-pounds per B.T.U. was
\, \frac {150 \times 33,000} {26.58 \times 180} = 1034.The ratio between the work performed and the heat generated thereby has come to be called the mechanical equivalent of heat. This is the first recorded estimate of the value of the mechanical equivalent of heat. It is too high by about one-third, the value accepted at present being \, 778 \frac { \text {ft.-lbs.} }{ \text {B.T.U} } = 4183 \frac { \text {joules} }{ \text {calorie} } .
Rumford’s value could scarcely be called a measurement. He did not measure the work done by the horse, and his precautions to avoid loss of heat were entirely inadequate. It is a wonder that he came as close to the correct value as he did. But the accuracy or lack of accuracy of the result is not the significant element in this experiment. The point most worthy of note is that in 1798, nearly fifty years before the idea of an equivalence between work and heat began to take its proper place in scientific doctrine, Count Rumford made a well-thought-out estimate of the numerical value of the numerical relation between the units of work and heat. Unfortunately he did not seem to appreciate the significance of his observation; for there is no record that he ever attempted to improve the accuracy of his measurement nor even that he gave the subject any further thought. Hence, the episode can only be regarded as one of those early, abortive stirrings of a great idea with which the history of science is filled.
The Next Step (page 296)
It would probably be correct to say that Rumford gave his energies to preliminary experimental work in a field the principal significance of which eluded him. When the field next begun to assume importance, forty-four years later, the situation was exactly reversed. The man who reopened the question in the next generation was, in contrast, enormously impressed with its significance; but his approach was characterized by an almost complete lack of experimentation. The subject was reopened by an article which appeared in a chemistry magazine, Liebig’s Annalen, in 1842, having been rejected by the principal physics publication of the day. It was written by an obscure German physician, J. R. Mayer (1818-78). Lacking both scientific education and opportunities for experimental work, Mayer was thus
“thrown back upon reflection, selecting with marvellous sagacity, from existing physical data, the single result on which could be founded a calculation of the mechanical equivalent of heat.”
The foregoing statement accompanied the belated award of the Copley medal to Mayer in 1871. The calculation of the mechanical equivalent of heat to which it refers was a part of Mayer’s paper of 1842. He had utilized some experimental data on the heat required to maintain the temperature of expanding air. The data themselves had long been known, but, again in the language of the award,
“No man, to my knowledge, prior to Dr. Mayer, penetrated the significance of these two numbers. He first saw that the excess was not, as then universally supposed, heat actually lodged in the (isothermally expanded) gas, but heat which had actually been consumed by the gas in expanding against pressure. The amount of work here performed was accurately known, the amount of heat consumed was also accurately known, and from these data Mayer determined the mechanical equivalent of heat.
Mayer Gets and Inspiration (page 297)
Mayer’s value, corrected for an erroneous value of the specific heat of air which he used was in English units \, 725 \, ft.-lbs per B.T.U. If this is compared with the correct value \, 778 , it may be recognized as a great improvement over Rumford’s \, 1034 . Yet this value was only a part and in one sense the lesser part of Mayer’s contribution. His great discovery, his great “hunch,” was what has since taken the form of the scientific doctrine of the Conservation of Energy. This was a concept toward which the scientific mind had been groping for two hundred years. In the 1842 paper he stated that
“force [the current form for energy] once in existence cannot be annihilated; it can only change its form.”
In this and in a succeeding paper in 1845 he expanded this idea to cover what was, especially for the time, an amazing variety of phenomena. He began with inorganic manifestations of interconvertibility of work and heat, including chemical reactions, and extended it to the brand-new idea that plants and coal deposits when burned were merely yielding up the heat previously received from the sun. He included animal life – which took in man – in his idea that heat and energy output are to be equated to intake, ultimately of energy from the sun. He extended the principle to astronomical phenomena, computing the speed of fall to the earth from an infinite distance as \, 34,450 \, feet per second, and proposed the contraction theory of the origin of the sun’s heat, both of these ideas being entirely new at that time (see 263).
The Opposition to Mayer and the Reason for It (297)
As a prophet in the scientific era Mayer may not have been unique, but he showed the attributes of a genius of the first order. It is sad to relate, therefore, that his life was a tragic one by the very virtue of his genius. The very insight which ultimately became the basis of his fame was at first the source of his misery. Since he was so far ahead of his time, recognition of his work was delayed, and he was treated as a freak or a madman. He attempted suicide and for a time became actually deranged. He recovered, however, and lived to experience and appreciate a considerable measure of the honor that was his due.
Every cultural group establishes its criterion of success, its “trumps,” and the game of life within that group must be played accordingly. In the “manly art,” for example, trumps is the knockout. A knockout automatically brings the championship, even though it may be demonstrable that the defeated contestant is unqualifiedly the more skillful of the two. In the legal profession, trumps is the winning of cases. That lawyer is the most successful who wins the largest number of decisions, even though his contentions may, aside from legal technicalities, be demonstrably contrary to fact.
The scientific world, like other cultural groups, has its trumps – the numerical agreement of experimental data. Such agreement in a given area of science is considered to be a vindication of the hypothesis leading up to the experiment in question. This confidence in numerical agreement may not be justified. After decades of such agreement some of the most basic scientific doctrines have been shown to be logically untenable. But the trumps still stand.
Against that background one may see why the scientific world disregarded Rumford’s work and at first poured contumely on Mayer’s. Though there were some elements in the theories of each man that were subject to experimental verification, the two men did not themselves provide such verification. One can understand too why when J. P. Joule, whose work will be considered shortly, verified numerically a few of Mayer’s pronouncements, a strong presumption was created in favor of, not merely the verified portion, but also all the rest of Mayer’s theory.
Joule Determines the Mechanical Equivalent of Heat (page 298)
James P. Joule (1818-89) was a brewer of Manchester, England. Like many other men of independent means in the history of science, he became a scientist by avocation. He first became interested in “electric engines,” the electric motor being in somewhat of a prenatal stage at that time. Before reaching his majority he had published several papers including, among other things, some measurements of the power developed by his rudimentary motors. In 1840 he presented a paper to the Royal Society on the production of heat by the electric current. It seems natural that, having measured both the power and the rate of evolution of heat manifested by an electric current, he should be led to compare the two. This he did, in 1842, the year of Mayer’s original paper. He deduced the value \, 838 \, ft.-lbs. per B.T.U. for the mechanical equivalent of heat. That was his beginning.
From that time on he devoted his life to redetermining, in every way that lent itself to experimental attack, the value of this constant. In 1843 he observed the rise in temperature due to the friction of water with the walls of capillary tubes through which it was driven, and deduced the value \, 770 . In 1845 he measured the heat developed or absorbed when air was compressed or expanded to the accompaniment of a measured amount of work
( 798 ). In the same year he observed the rise in temperature of water churned by a paddle wheel whose work was measured ( 890 ). Dissatisfied with this he repeated the same measurement in oil in 1847 ( 781.5 \, and 782.1 ). In 1849 he repeated the same measurements
( 772 ). In 1850 he agitated mercury ( 774 ). In the same year he utilized friction in cast iron
( 775 ). In 1867 he heated water electrically instead of by a paddle wheel ( 783 ). In 1878 he repeated his paddle wheel experiment and deduced his last value, 772.55 .
Joule concluded one of his papers with the following statement:
“I will therefor conclude by considering it as demonstrated by the experiments contained in this paper – (1) that the quantity of heat produced by the friction of bodies, whether solid or liquid is always proportional to the quantity of force [i.e., work] expended;
and (2) that the quantity of heat capable of increasing the temperature of a pound of water (weighed in vacuo, and taken at between \, 55° and \, 60° ) by \, 1° Fahr., requires for its evolution the expenditure of a mechanical force [i.e., work] represented by the fall of \, 772 \, pounds through the space of \, 1 \, foot.”
Joule’s preoccupation with the measurement of the mechanical equivalent of heat is illustrated by a story told by Lord Kelvin, one of Joule’s friends and scientific associates. Shortly after their first meeting – which occurred, it afterwards developed, three days before Joule’s marriage – Kelvin, who was vacationing in Switzerland, saw a young man approaching him, gingerly carrying something resembling a walking stick. It proved to be Joule, on his honeymoon, carrying a large thermometer which he had made in order to measure the temperature of the water above and below an eight-hundred-foot waterfall which he and his bride expected to visit (278:54-56).
Joule and the Conservation Concept (page 300)
Joule, like Mayer, was animated by a conviction of the conservation of energy. Though he did not particularize on his theory as Mayer did, his conception of the inclusiveness of the doctrine was no less wide. In his 1843 paper he said (165:205):
“I shall lose no time in repeating and extending these experiments, being satisfied that the grand agents of nature are, by the Creator’s fiat, indestructible; and that whenever mechanical force [i.e., work] is expended, an exact equivalent of heat is always obtained.”
The italics are Joule’s own. This statement is no less inclusive than Mayer’s. Indeed because he did not particularize as Mayer did, it may be said to be broader. A comparison of the inclusiveness of this statement with the very limited number of ways that the principle lent itself to verification at his hands will make it clear that his experiments constituted far from conclusive evidence on the validity of his belief. Yet because he performed experiments, even experiments which had little to do with some of the aspects of his doctrine, his doctrine was accepted in its entirety.
The later acceptance of Mayer’s doctrine came only when it was seen to be identical of Joule’s. Actually, the volume of qualitative evidence covering a wide variety of phenomena which Mayer proffed constituted better support of the broad implications of the doctrine of conservation of energy than did the close numerical agreement of Joule’s results in a limited field. But Joule held the scientific trumps.
Not that Joule’s ideas commanded immediate assent, however. Leading men of science, though interested in Joule’s work, were dubious about its correctness for several years. Some of them based their incredulousness on the nature of Joule’s experiments, remarking that he “had nothing but hundredth of a degree to prove his case by.” Others considered Joule’s doctrine incompatible with a theory of Carnot about heat transformations, which will be considered in the following chapter. Carnot’s theory held a position of dominance at that time, and all other theories of heat that failed to agree with it were under suspicion. There are ephemeral modes of thought in science as well as in other walks of life.
The New Principle of Conservation of Energy (page 301)
It is perhaps unnecessary to emphasize the new “turn” that the doctrine of Mayer and of Joule gave to the old principle of conservation of energy. The old form was restricted to purely mechanical transformations. The phenomena of oscillation, of elastic impact, and of frictionless fluid motion are frequently treated as in Chapters 15, 16, and 17, with the aid of the restricted form of the principle. But wherever friction or other types of dissipation of energy are involved, accompanied as they must ultimately be by the generation of heat, only the more inclusive form of the conservation principle is adequate. That form is variously phrased, but the following statement is perhaps as good as any (211:118):
“Energy is recognized in various forms, and when it disappears in one form it appears in others, and in each case according to a fixed rate of exchange. The total quantity of any energy, measured in terms of any one form, is constant whatever forms it may assume.”
Many years were to elapse before the scientific world came to a general recognition of the full import of the doctrine of conservation of energy. Perhaps the most effective of its earlier champions was Hermann von Helmholtz, who was at that time (1847) principally known as a physiologist who was especially versed in the field of sound.
It is somewhat thought-provoking to realize that the five men who were the first to comprehend the full import of the principle of the conservation of energy were all young men and were all professionally outside of the field of physics at the time that they made their contributions. These were Mayer, a German physician, aged twenty-eight; Carnot, a French engineer who preceded all the rest in the discovery and who will be discussed further in the next chapter, aged thirty-four; Helmholtz, a German physiologist, aged thirty-two, Joule, and English industrialist, aged twenty-five; and Colding, a Danish engineer who made the same discovery independently of the others and almost simultaneously, aged twenty-seven.
Perpetual Motion (page 301)
It was the principle of conservation of energy, a principle which Poincaré has termed “the grandest conquest of contemporary thought,” that finally set in strong relief the futility of the perennial efforts to devise a perpetual motion machine. A corollary of the principle that that the sum of all the forms of energy output of a machine must always be exactly equal to the total energy input is that the output can never exceed the input. What constitutes the will-o’-the-wisp of the perpetual motionist has been the fond hope in many quarters that a machine could be devised whose output would be greater than its input. The principle of conservation of energy alone, therefore, should be enough to dispel this illusion.
Actually the case for perpetual motion is even less valid than the conservation principle, taken by itself, allows. The statement that the total energy output of a machine is always equal to the input includes in the energy output such items as friction and heat loss, inescapable characteristics of the operations of any machine. These forms of “output” are useless whereas the perpetual motionist is naturally interested only in useful output. The useful output of any machine is consequently always less than the input, the ratio of the two being termed efficiency, a term already introduced in the study of mechanics (page 230).
The efficiency of common machines is much lower than the average individual realizes. That of a high quality internal combustion engine is about 40 per cent, of a high quality steam engine about 20 per cent, of a locomotive engine about 5 percent. There are, moreover, certain losses in the transmission of mechanical or electrical energy to the locality of utilization and in its application at the point of consumption which diminish practical efficiency still further. When coal is burned to supply steam for the generation of electricity, only a small fraction of 1 percent of the coal’s heat energy appears in the illumination provided by the electricity. All the rest is lost along the way, dissipated in the form of heat. Even the light itself is absorbed and ultimately converted into heat.
The Degradation of Energy (page 302)
The same can be said of any series of energy transformations whatever. There is a “tax,” payable only in the form of heat, exacted at each step. All kinds of energy ultimately dissipate themselves in the form of heat. The tragedy of the process is that the heat thus generated can practically never be utilized. Once dissipated it can never be recovered or reclaimed. The user can only turn to the source, almost invariably the sun in the final analysis, for another handout, which will be similarly spent in its turn. Just as much energy resides in the dissipated heat as resided in the original handout from the sun, but it has been rendered unavailable. The process is technically termed the degradation of energy.
The degradation of energy was first identified by Lord Kelvin in 1851 and is perhaps as important a scientific generalization as is the conservation of energy. Kelvin pointed out that, as a consequence of this continuous universal process, the inescapable decreasing availability of energy indicates an ultimate stoppage of all energy flow in the universe, a “running down” of the cosmic clock. Long before that state is reached all life will have disappeared, for life depends on, and perhaps consists of, a peculiar form of energy flow. The end of the world is usually pictured as a state of utter frigidity.
This is not necessarily a correct picture. The final state of the universe may be temperate or even hot. The important point will not be temperature level, but the entire absence of differences of temperature. Everything will be cold or medium or hot to the same degree, and consequently no energy can flow from one place to another. This state of affairs is inevitable, according to every evidence now available. Perhaps it is not necessary to take the matter too seriously, however, for even the most enthusiastic prophets of doom admit that many billions of years will elapse before that condition comes to pass, and a lot of things can happen in a billion years.
Heat Engines (Chapter 23)
Work as a Form of Heat (page 304)
The usual conception of an “engine” as a source of power places at the center of the stage the picture of a great force: the force of expanding steam in the steam engine or of explosively burning vapors in the case of the internal combustion engine. Of course it is true that sources of commercial power do exert forces, yet those forces would be useless if their exertion did not result in motion. It is the rate at which an engine does work that counts – not primarily the force that it exerts. But whatever the immediate manifestation of work, the ultimate origin can only be a transfer of heat, a portion of which incidentally becomes converted into work. This idea was emphasized in the preceding chapter and will be further developed in this one. It suggests the reason why all devices for converting stores of heat into mechanical work are termed heat engines. It indicates why James Watt’s first contribution to the development of the steam engine – which was the prevention of a great waste of heat – was also his greatest contribution.
Watt’s First Improvement of the Steam Engine (page 304)
The old Newcomen engine which had been turned over to Watt for repair in 1763 even at its best was consuming at each stroke several times as much steam as was needed merely to fill the cylinder. This was because the steam came in contact with the cylinder walls, which were chilled from the jet of water that had condensed the previous cylinder-full, and most of it went into heating the walls up to its own temperature. So much was being lost in this way that Watt found that the engine was consuming at every stroke eight cylinder-fulls of steam. Watt, in collaboration with Black, had experimented extensively on the “latent heat” of steam, and he was acutely aware of the huge amount of heat which was being lost. It took him a long while, however, to see how it could be avoided.
After considering the possibility of making cylinders of some material of low specific heat, which would, therefore, warm up with little heat, he finally hit upon a much better idea. He is very explicit about the time and place where this came to him. He says that he was walking on the Glasgow Green on a spring day in 1765 thinking of the engine,
“when the idea came into my mind that, as steam was an elastic body, it would rush into a vacuum, and, if a communication were made between the cylinder and an exhausted vessel, it would rush into it, and might be there condensed without cooling the cylinder.”
Watt’s idea of providing a separate condenser effected a greater improvement in the economy of steam-engine operation than any other invention – possibly than all others combined. It has been characterized as (163:122):
“probably the most important technical advance of the eighteenth century and one that may be truly said to have ushered in a new era of industrial development.”
Important as was this discovery of Watt’s, it was more of a technological than a scientific advance. In some of his later work these relative emphases were reversed.
Utilizing the Expansive Energy of Steam (page 306)
In all of the early engines, including those benefiting from Watt’s first improvement, steam at full boiler pressure followed the piston clear to the end of its travel. When the exhaust valve was opened at the end of the stroke, this high-pressure steam escaped without doing any further work. This was another source of wastage to which Watt some years later turned his attention. He solved this problem by shutting the steam off early in the stroke and allowing it to expand, a principle which has characterized the operation of steam engines ever since. Under this arrangement the pressure in the cylinder falls gradually during the last portion of each stroke, almost or quite to that of the condenser. This greatly reduces the loss of power at each exhaust.
As a current example of little or no expansion we may consider a locomotive engine starting under a heavy load. The loud sound of its exhaust under these circumstances shows the wasted energy. Such sound, on the other hand, is almost entirely absent from a high-quality stationary engine. This performance is approached, indeed, by the locomotive engine as it picks up speed and the cut-off is progressively shifted to points earlier in the stroke.
The Pressure-Volume Diagram (page 306)
[…]
The Indicator Diagram (page 307)
One of Watt’s great contributions to the development of the steam engine was the devising of an instrument called a steam engine indicator, which would record just such diagrams for engines in actual operation. Indicator diagrams, so called, have been of immense utility too engineers ever since. They not only give information on the power developed by an engine, that is work per stroke multiplied by numbers of stroke per second, but – what is even more useful – disclose the faults in adjustment of the valve gear and give other types of information on the working condition of an engine. Thus in Fig. 155 the faults disclosed by each diagram are indicated. It would be very difficult to detect these faults in any other way.
Of Watt’s other numerous improvements of the steam engine, the most notable is making engines double acting; that is, applying the steam first to one side and then to the other of the piston, instead of limiting it to one side as in all engines up to 1782. All in all, Watt’s work was so far-reaching that for a century after his death further improvements in the steam engine could only be made in the details.
The Steam Engine Goes Scientific (page 308)
But the impossibility of surpassing Watt in improving the practical operation of the steam engine did not preclude the possibility of deepening the scientific comprehension of the principles involved. This was the next step, and it was taken by a brilliant young French engineer, S. N. L. Carnot (1796-1832), in 1824. By that time the steam engine had become very common in industrial practices, though it had not yet been applied extensively to transportation. The Atlantic had been crossed only once under steam power (1819), and the locomotive had not yet come into existence. Carnot sensed that the theory of the steam engine was comprehended vaguely or not at all, and set himself the task of correcting this. In introducing the Reflections on the Motive Power of Heat, his only publication, he remarked (165:3-5):
“It is well known that heat may be used as a cause of motion, and that the motive power which may be obtained from it is very great. The steam engine, now in such general use, is a manifest proof of this fact…. The study of the steam engine is of the highest interest, owing to its importance, its constantly increasing use, and the great changes it is destined to make in the civilized world….
In spite of labor of all sorts expended on the steam engine, and in spite of the perfection to which it has been brought, its theory is very little advanced, and the attempts to better this state of affairs have thus far been directed almost at random.”
Carnot’s great contribution was in directing attention to the fact that the real source of “motive power” was difference of temperature. The exertion of pressure on a piston was an incidental detail of a complicated process, the heart of which was the flow of heat. He said (164:7-9):
“The production of motion in the steam engine is always accompanied by a circumstance which we should particularly notice. The circumstance is the passage of caloric from one body where the temperature is more or less elevated to another where it is lower. What happens, in fact, in a steam engine at work?
The caloric developed in the fire-box as an effect of combustion passes through the wall of the boiler and produces steam, incorporating itself with the steam in some way. This steam carrying the caloric with it, transports it first into the cylinder, where it fulfills some function, and then into the condenser, where the steam is precipitated by coming into contact with cold water. As a last result, the cold water in the condenser receives the caloric developed by combustion. It is warmed by means of the steam as if it had been placed directly in the fire-box.
Everywhere where there is a difference in temperature, and where (a flow of) caloric can be effected, the production of motive power is possible. Water vapor is one agent for obtaining this power, but it is not the only one; all natural bodies can be applied to this purpose, for they are all susceptible to changes of volume, to successive contractions and dilations effected by alternations of heat and cold; they are all capable, by this change of volume, of overcoming resistances and thus of developing motive power…. The vapors of all bodies which are capable of evaporation, such as alcohol, mercury, sulphur, etc., can perform the same function as water vapor.
The Steam Engine as a Heat Engine (page 310)
Carnot’s extension of the list of substances capable of utilization in power production is interesting in view of subsequent developments. He did not cite gasoline, a substance unknown in his day, but he did mention alcohol, the use of which is on the increase, and mercury, which was first used for such a purpose only a little over a decade ago.
The emphasis which Carnot thus placed on the central rôle played by the transfer of heat in the operation of what he termed “heat engines” was of the utmost significance. He thereby put his finger on the crucial element in all engines, an idea which seemed to have occurred to no one before him. He was most explicit about it (165:20-21):
“The motive power of heat is independent of the agents employed to develop it; its quantity is determined solely by the temperatures of the bodies between which, in the final result x8i.e., upon the completion of a cycle of operations] the transfer of the caloric occurs.”
The idea was of even broader import than Carnot realized at the time, for he specifically excepted machines “worked by men or animals, by waterfalls or by air currents.” Not possessing the concept of the mechanical equivalent of heat, he was in no position to realize that these also, “in the final result,” were subject to his great generalization that “motive power” was always attributable to heat transfer.
Overlooking the Conservation of Energy (page 310)
Carnot made another error connected with his association of motive power with heat transfer. It was much more serious than his simple error of omitting engines which were not apparently of the heat-transfer type. The more serious error arose, as did the lesser error, because he did not possess the concept of the mechanical equivalent of heat. Out of his preoccupation with the flow of heat in connection with engines he jumped to the conclusion that the theoretically perfect engine would simply transfer all the heat that it received from the source of higher temperature, intact to the region of lower temperature. He used an analogy which was quite plausible.
“The production of motive power in the steam-engine is therefore not due to a real consumption of the caloric, but its transfer from a hotter to a colder body…. We may with propriety compare the motive power of falling water depends on the quantity of caloric employed and on that which might be named, which we, in fact, will call its descent – that is to say, on the difference of temperature of the bodies between which the exchange of caloric is effected.”
Conservation Comes In (page 311)
The italics in the foregoing equation are Carnot’s own. His idea was perfectly clear and his analogy rather convincing. But it was wrong. We now know that a part of the heat supplied to any engine is diverted and changed to mechanical energy, so that less heat is delivered to the exhaust of even a perfect engine that is supplied from the source. Subsequent measurements (128) showed that the heat delivered at the exhaust of a steam engine was less than that supplied to it, the difference being the heat equivalent of the work done by the engine (see Fig. 157).
The principle of conservation of energy makes this conclusion mandatory for us, but that principle was unknown in 1824. It was, in fact, the apparent contradiction between Carnot’s theory and the doctrine promulgated twenty years later by Mayer and by Joule which was primarily responsible for the doubts with which the latter was regarded. This error of Carnot’s is another misdemeanor which must be chalked up to the discredit of the caloric theory of heat. It was his commitment to this theory which caused Carnot to be impressed by the analogy between the waterfall and the heat-engine and which lead him to identify his of a sort of “conservation of heat” with the doctrine of conservation of matter, which had been experimentally established twenty years before.
Though this was Carnot’s influence on the development of scientific doctrine, he apparently changed his opinion before his death in 1832. Some of his later papers, published posthumously in 1872 in connection with a second edition of his Reflections, make it unmistakable that he had acquired a clear prevision of the principle of conservation of energy; that he had even made a surprisingly accurate determination of the mechanical equivalent of heat; and that he had laid out for himself a program of investigation which included all the important developments in this field made by others in the ensuing thirty years, a truly amazing foresight.
If he had not been cut down during an epidemic of cholera at the age of thirty-six, he would probably have developed into one of the greatest men of science of all time.
The Reversibility of a Engine Cycle (page 312)
Carnot discovered another attribute of engines which was only second in importance to his emphasis on their heat relations, namely their reversibility. This does not refer to anything as trivial as the direction of rotation. What it refers to is the possibility of actually reversing the operation which an engine normally performs. In normal operation an engine produces mechanical energy by taking heat from a region of high temperature and delivering some of it to one of low. Therefore, when “reversed,” it should be capable of absorbing mechanical energy to effect a transfer of heat from a region of low temperature to one of high. Carnot envisioned this with his remark that
“wherever there is a difference of temperature the production of motive power is possible.”
He added,
“Conversely, whenever this power can be employed, it is possible to produce a difference of temperature…. To effect this the operations which we have just described could have been performed in a reverse sense and order.”
The Principle of the Mechanical Refrigerator (page 313)
The process (represented in Fig. 158) is simply the reverse of that pictured in the right-hand portion of Fig. 157. Energy is furnished to the system at the point marked “motive power input.” This “pumps” heat from the cold region to the hot region, adding its own heat equivalent to the quantity thus delivered to the region of high temperature. This is simply the working principle of the ordinary electric refrigerator. The cooling unit is the “low temperature” part of Fig. 158. Heat is pumped out of it by the compressor, “motive power input,” into the condenser, “high temperature,” and thence escapes into the room outside the refrigerator. Thus the compressor of such a refrigerator is simply an engine with its action reversed.
The Mechanical Refrigerator in Practice (page 313)
Fig. 160 shows the operation of one of the two types of refrigerator now in common domestic use, the compression type. As in all types, the refrigeration is produced by the evaporation of a volatile liquid in the evaporator or “cooling unit.” The resulting vapor is then pumped up to high pressure by a motor-driven compressor. The high-pressure vapor is condensed back into a liquid, usually by an air-cooled “condenser.” It is at this point that the heat removed from the interior of the refrigerator plus the heat equivalent of the work done by the electric motor on the compressor is delivered to the room. The liquid then enters the cooling unit, is vaporized and the cycle is repeated.
Fig. 161 shows the operation of the other type of mechanical refrigerator in common domestic use, the absorption type. This type differs from the former principally in the agency used to remove the vapor from the evaporator, condense it back to a liquid, and then complete the cycle. The two processes are identical as far as what occurs in the cooling unit is concerned, the evaporator being the actual point of application of refrigeration, whatever the system.
The absorption type of refrigerator uses a refrigerant – ammonia – which is readily soluble in water. A convection current, maintained by a flame – usually of gas, whence the name “gas refrigerator” – carries the ammonia to the absorber, where it is dissolved into water. The solution then flows to the generator, where the ammonia is boiled out of the water again. The water, raised by the agency of the heat through a process made familiar in coffee percolators, flows back into the absorber. The hot ammonia vapor rises to the condenser where, after being converted back into a liquid, it flows by gravity back to the evaporator, thus completing the cycle. The cycle of events is aided by the presence of a gas – hydrogen – under a pressure of several hundred pounds, which is sealed into the system, along with the ammonia and water, in the process of manufacture of this type of refrigerator.
To the uninitiated the spectacle of “cooling by heat” appears paradoxical. It is a “paradox,” however, which is equally characteristic of the “electric” and the “gas” types of refrigerator. In both cases energy is required to lift the heat out of the refrigerator and deliver it to the higher temperature of the surroundings. Whether the energy is furnished in the form of electricity or heat is an incidental detail. If the system is of a type which requires heat, heat could be furnished equally well by electricity or gas. If it requires mechanical energy, that too could be provided either by an electric motor or, say, by a steam engine supplied from a boiler by gas. The central point in any type of refrigerator is the element foreseen by Carnot, the absorption of energy involved in the engine cycle when reversed.
Refining the Concept of Reversibility (page 315)
Yet the concept of reversibility in its full sweep involves more than merely reversing the function of an engine. It requires that the engine shall be theoretically perfect, without friction, and work without any heat losses or other dissipation of energy. For energy so lost cannot be recovered and a machine which incurred such losses could never be reversible in this way, even if the engine were perfectly insulated against heat losses. In fact special precautions would have to be observed to make it reversible.
For example, if at the end of its stroke the pressure of the expanded gas is greater than that in the condenser, as in most of the diagrams of Fig. 155, it escapes with a characteristic “puff” as the exhaust valve is opened, and this constitutes an irreversible event. A moment’s thought will make it clear that the attempt to reverse such an event would be unsuccessful. For when the intake valve of a compressor closes and compression is about to begin, the gas could not be expected to raise its own pressure spontaneously before compression actually begins, to the extent that it dropped its pressure at the same point previously (Fig. 162).
There are other ways in which a cycle can be rendered irreversible and most of them get in their work in practice, especially in the innumerable ways in which heat can be lost to the surroundings in effecting a cycle of such operations. But in theory, the reversible cycle is a possibility of which much has been made, entirely aside from its utility in the mechanical refrigerator. It will be utilized in the present chapter.
The Efficiency of a Heat Cycle (page 316)
The efficiency of any operation has been defined (page 230) as the ratio of useful energy yielded to total energy absorbed. Originally formulated to apply to merely mechanical operations, it is still a valid concept when heat and other forms of energy are included. Specifically, it is applicable to the engines which have been under discussion. A certain quantity of heat, \, Q_1 , leaves the high temperature source in connection with the operation of an engine. Part of it, \, Q_2 , reaches the low-temperature reservoir, mainly through the exhaust. Assuming no heat losses or other dissipations of energy, the difference \, (Q_1 - Q_2) , represents the quantity of heat converted into mechanical energy by the engine. The efficiency of the process is
\, \text {eff.} = \dfrac {Q_1 - Q_2}{Q_1}. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (1)
But the italicized assumption is the condition that the process shall be reversible. Hence equation (1) represents the efficiency of a reversible cycle. If the assumption has been incorrect, part of \, Q_1 \, being dissipated, then the energy yielded by the engine will be less than \, (Q_1 - Q_2) \, by the amount so lost and the efficiency less than that represented in equation (1). So equation (1) represents the maximum efficiency that any engine can have. The assertion that no engine can be more efficient than a reversible engine is known as Carnot’s theorem.
Carnot deduced his theorem in different terms, however. He derived it from his water-power analogy (page 311), picturing motive-power as depending on
“the quantity of caloric and on the … difference of temperature,”
considering it proportional to the product. Hence, taking \, Q \, as the quantity of heat descending – on Carnot’s view – from a region of absolute temperature \, T_1 \, to a region of
absolute temperature \, T_2 , the work actually done was proportional to the product \, Q (T_1-T_2) \, and the maximum work theoretically obtainable was \, Q T_1 . Hence the efficiency was
\, \text {eff.} = \dfrac { Q (T_1 - T_2) }{ Q T_1 }. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (2)
Though this expression, like Black’s concepts of specific and “latent” heat, was deduced on a false premise about the nature of heat, it was subsequently found, also like Black’s results, to be nevertheless correct. Hence, the efficiency of a reversible cycle is expressible by either equation (1) or equation (2). This furnished the real basis for Carnot’s generalization quoted on page 310.
/////// End of Quote from Taylor
/////// Quoting Taylor (page 317)
A Basic Scale of Temperatures (page 317)
Comparison of equations (1) and (2) shows that
\, \dfrac {T_1} {T_2} = \dfrac {Q_1} {Q_2}. \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\; (3)
Thus two temperatures on this scale are to each other as the heats absorbed and ejected respectively by a perfectly reversible engine operating between heat reservoirs at these temperatures. The comparison of temperatures in this way, though depending on the imaginary operation of a perfectly reversible engine, is, by the principles of such an engine, independent of the properties of any particular substance. Comparison of temperatures in the conventional manner, on the contrary, is inescapably dependent on the properties of particular substances, usually on their expansivities or their pressure coefficients.
The scientific mind seems to have been greatly compforted at finding this “absolute” method of comparing temperatures. It was first developed by Lord Kelvin in 1848, and the scale which was established on this basis has become known as the Kelvin absolute scale. It is exceedingly close to the scale of absolute temperatures as already described in Chapter 19 (page 254), and it is because of this association with Kelvin that the notation \, K. \, is used for absolute temperatures just as \, F. \, and \, C. \, are used for Fahrenheit and centigrade respectively.
The Kelvin scale of temperature leads also to another notion of absolute zero. The efficiencies of ordinary engines are always less than unity. But inspection of equations (1) and (2) will show that the efficiency of a perfectly reversible engine could attain the value unity, provided that \, Q_2 \, (as also \, T_2 ) possessed the value zero. In this case all the heat, \, Q_1 , taken in from the source would be converted into work. Since we cannot suppose that more work can be evolved than is represented by the heat drawn from the source, a negative value of \, T \, is impossible, and, hence, the temperature corresponding to \, T = 0 \, is the lowest conceivable. The best present value of this in terms of the centigrade scale is \, -273.18° C. A temperature within \, .005 \, degree of this point has actually been reached. If the improvement of low-temperature technique should ultimately result in the attainment of temperatures any lower than the supposed value of the absolute zero it would constitute a somewhat embarrassing accomplishment.
[…]
The Significance of Industrial Power (page 323)
A great deal more could undoubtedly be said with profit on the subject of current engineering practice, but it would be too far afield from the objectives of this chapter and indeed of this book. As Carnot himself remarked at one point (164:54):
“We shall say nothing more on this subject, our object not being to discuss the details of construction of heat-engines. These should be treated in a separate work.”
The important point is not current engineering practice, nor even those aspects of it that were foreseen by the geniuses of a century and more ago. It is rather the foundation that they laid, partly unwittingly, for a new point of view in physical science, for the industrial era which created modern material civilization, and for the new way of thinking about things that is the principal distinction between modern and ancient society. As the German chemist Liebig said in 1866:
“The progress of Greek civilization was dependent essentially on the change of slave-labor into free, a transformation not supposable without the employment of natural forces, applied to labor-saving machines…. Only the free man, not the slave, has a disposition and interest to improve implements or to invent them…. The improvement of established industrial methods by slaves, themselves industrial machines, is out of the question.”
/////// End of Quote from Taylor
• History of Clausius’ theorem